“In summary, these results suggest that psychoactive cannabinoid exposure during adolescence is unlikely to have a major effect on the escalation of cocaine intake or the development of compulsive-like responding per se in adulthood in a rat model of cocaine self-administration.”
Monthly Archives: September 2018
Benefits and Risks of Therapeutic Cannabinoids for Neurologic Disorders

“The Cannabis genus originated in Central Asia and is probably one of the most ancient nonfood crops to be cultivated by humans. Its medicinal properties have been recognized for centuries. Isolation of the psychoactive compound, Δ9-tetrahydrocannabinol, followed by the identification of cannabidiol, led to increased focus on the therapeutic potential of the plant. One of the prominent species, Cannabis sativa, may produce more than 100 different cannabinoids.”
https://www.ncbi.nlm.nih.gov/pubmed/30224192
https://www.clinicaltherapeutics.com/article/S0149-2918(18)30331-X/fulltext
Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor.

“Cannabidiol (CBD), the major non-psychotomimetic compound present in the Cannabis sativa plant, exhibits therapeutic potential for various human diseases, including chronic neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, ischemic stroke, epilepsy and other convulsive syndromes, neuropsychiatric disorders, neuropathic allodynia and certain types of cancer.
CBD does not bind directly to endocannabinoid receptors 1 and 2, and despite research efforts, its specific targets remain to be fully identified. Notably, sigma 1 receptor (σ1R) antagonists inhibit glutamate N-methyl-D-aspartate acid receptor (NMDAR) activity and display positive effects on most of the aforesaid diseases. Thus, we investigated the effects of CBD on three animal models in which NMDAR overactivity plays a critical role: opioid analgesia attenuation, NMDA-induced convulsive syndrome and ischemic stroke.
In an in vitro assay, CBD disrupted the regulatory association of σ1R with the NR1 subunit of NMDAR, an effect shared by σ1R antagonists, such as BD1063 and progesterone, and prevented by σ1R agonists, such as 4-IBP, PPCC and PRE084. The in vivo administration of CBD or BD1063 enhanced morphine-evoked supraspinal antinociception, alleviated NMDA-induced convulsive syndrome, and reduced the infarct size caused by permanent unilateral middle cerebral artery occlusion.
These positive effects of CBD were reduced by the σ1R agonists PRE084 and PPCC, and absent in σ1R-/- mice. Thus, CBD displays antagonist-like activity toward σ1R to reduce the negative effects of NMDAR overactivity in the abovementioned experimental situations.”
https://www.ncbi.nlm.nih.gov/pubmed/30223868
https://molecularbrain.biomedcentral.com/articles/10.1186/s13041-018-0395-2
Emerging Evidence for Cannabis’ Role in Opioid Use Disorder.
 “The opioid epidemic has become an immense problem in North America, and despite decades of research on the most effective means to treat opioid use disorder (OUD), overdose deaths are at an all-time high, and relapse remains pervasive.
“The opioid epidemic has become an immense problem in North America, and despite decades of research on the most effective means to treat opioid use disorder (OUD), overdose deaths are at an all-time high, and relapse remains pervasive.
Although there are a number of FDA-approved opioid replacement therapies and maintenance medications to help ease the severity of opioid withdrawal symptoms and aid in relapse prevention, these medications are not risk free nor are they successful for all patients. Furthermore, there are legal and logistical bottlenecks to obtaining traditional opioid replacement therapies such as methadone or buprenorphine, and the demand for these services far outweighs the supply and access.
To fill the gap between efficacious OUD treatments and the widespread prevalence of misuse, relapse, and overdose, the development of novel, alternative, or adjunct OUD treatment therapies is highly warranted. In this article, we review emerging evidence that suggests that cannabis may play a role in ameliorating the impact of OUD. Herein, we highlight knowledge gaps and discuss cannabis’ potential to prevent opioid misuse (as an analgesic alternative), alleviate opioid withdrawal symptoms, and decrease the likelihood of relapse.
Conclusion: The compelling nature of these data and the relative safety profile of cannabis warrant further exploration of cannabis as an adjunct or alternative treatment for OUD.”
Exploring the Ligand Efficacy of Cannabinoid Receptor 1 (CB1) using Molecular Dynamics Simulations.
“Cannabinoid receptor 1 (CB1) is a promising therapeutic target for a variety of disorders. Distinct efficacy profiles showed different therapeutic effects on CB1 dependent on three classes of ligands: agonists, antagonists, and inverse agonists. To discriminate the distinct efficacy profiles of the ligands, we carried out molecular dynamics (MD) simulations to identify the dynamic behaviors of inactive and active conformations of CB1 structures with the ligands. In addition, the molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) method was applied to analyze the binding free energy decompositions of the CB1-ligand complexes. With these two methods, we found the possibility that the three classes of ligands can be discriminated. Our findings shed light on the understanding of different efficacy profiles of ligands by analyzing the structural behaviors of intact CB1 structures and the binding energies of ligands, thereby yielding insights that are useful for the design of new potent CB1 drugs.”
https://www.ncbi.nlm.nih.gov/pubmed/30213978
https://www.nature.com/articles/s41598-018-31749-z
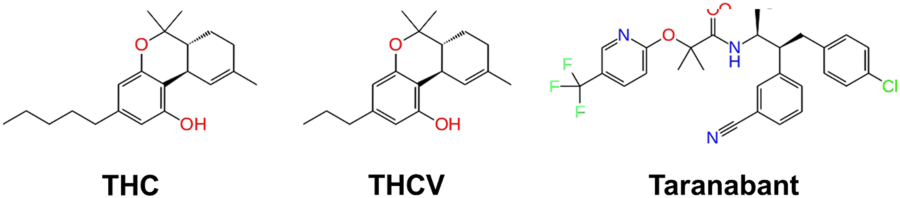
“Chemical structure of the partial agonist THC, antagonist THCV, and inverse agonist Taranabant.”
Role of the endocannabinoid system in drug addiction.

“Drug addiction is a chronic relapsing disorder that produces a dramaticglobal health burden worldwide. Not effective treatment of drug addiction is currently available probably due to the difficulties to find an appropriate target to manage this complex disease raising the needs for further identification of novel therapeutic approaches.
The endocannabinoid system has been found to play a crucial role in the neurobiological substrate underlying drug addiction.
Endocannabinoids and cannabinoid receptors are widely expressed in the main areas of the mesocorticolimbic system that participate in the initiation and maintenance of drug consumption and in the development of compulsion and loss of behavioral control occurring during drug addiction.
The identification of the important role played by CB1 cannabinoid receptors in drug addiction encouraged the possible used of an early commercialized CB1 receptor antagonist for treating drug addiction.
However, the incidence of serious psychiatric adverse events leaded to the sudden withdrawal from the market of this CB1 antagonist and all the research programs developed by pharmaceutical companies to obtain new CB1 antagonists were stopped.
Currently, new research strategies are under development to target the endocannabinoid system for drug addiction avoiding these side effects, which include allosteric negative modulators of CB1 receptors and compounds targeting CB2 receptors.
Recent studies showing the potential role of CB2 receptors in the addictive properties of different drugs of abuse have open a promising research opportunity to develop novel possible therapeutic approaches.”
https://www.ncbi.nlm.nih.gov/pubmed/30217570
https://www.sciencedirect.com/science/article/abs/pii/S0006295218303952
False-positive cannabinoid screens in adult cystic fibrosis patients treated with lumacaftor/ivacaftor

“Cystic fibrosis (CF) is caused by gene mutations resulting in defective cystic fibrosis transmembrane conductance regulator (CFTR) protein activity. CFTR modulators have been developed to improve CFTR protein function. The combination of ivacaftor (IVA) and lumacaftor (LUM) partially restores CFTR protein function of F508del, the most common CF mutation.”
https://www.ncbi.nlm.nih.gov/pubmed/30217546
“False-positive cannabinoid screens in adult cystic fibrosis patients treated with lumacaftor/ivacaftor”
https://www.cysticfibrosisjournal.com/article/S1569-1993(18)30754-9/fulltext
Endocannabinoids in the treatment of gasytrointestinal inflammation and symptoms.

“The evolving policies regarding the use of therapeutic Cannabis have steadily increased the public interest in its use as a complementary and alternative medicine in several disorders, including inflammatory bowel disease.
Endocannabinoids represent both an appealing therapeutic strategy and a captivating scientific dilemma.
Results from clinical trials have to be carefully interpreted owing to possible reporting-biases related to cannabinoids psychotropic effects. Moreover, discriminating between symptomatic improvement and the real gain on the underlying inflammatory process is often challenging.
This review summarizes the advances and latest discovery in this ever-changing field of investigation, highlighting the main limitations in the current use of these drugs in clinical practice and the possible future perspectives to overcome these flaws.”
https://www.ncbi.nlm.nih.gov/pubmed/30218940
https://www.sciencedirect.com/science/article/pii/S1471489218300183?via%3Dihub
Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors.

“The chronic use of drugs that reduce the dopaminergic neurotransmission can cause a hyperkinetic movement disorder called tardive dyskinesia (TD). The pathophysiology of this disorder is not entirely understood but could involve oxidative and neuroinflammatory mechanisms.
Cannabidiol (CBD), the major non-psychotomimetic compound present in Cannabis sativa plant, could be a possible therapeutic alternative for TD. This phytocannabinoid shows antioxidant, anti-inflammatory and antipsychotic properties and decreases the acute motor effects of classical antipsychotics.
The present study investigated if CBD would attenuate orofacial dyskinesia, oxidative stress and inflammatory changes induced by chronic administration of haloperidol in mice. Furthermore, we verified in vivo and in vitro (in primary microglial culture) whether these effects would be mediated by PPARγ receptors.
The results showed that the male Swiss mice treated daily for 21 days with haloperidol develop orofacial dyskinesia. Daily CBD administration before each haloperidol injection prevented this effect.
Mice treated with haloperidol showed an increase in microglial activation and inflammatory mediators in the striatum. These changes were also reduced by CBD. On the other hand, the levels of the anti-inflammatory cytokine IL-10 increased in the striatum of animals that received CBD and haloperidol.
Regarding oxidative stress, haloperidol induced lipid peroxidation and reduced catalase activity. This latter effect was attenuated by CBD. The combination of CBD and haloperidol also increased PGC-1α mRNA expression, a co-activator of PPARγ receptors. Pretreatment with the PPARγ antagonist, GW9662, blocked the behavioural effect of CBD in our TD model. CBD also prevented LPS-stimulated microglial activation, an effect that was also antagonized by GW9662.
In conclusion, our results suggest that CBD could prevent haloperidol-induced orofacial dyskinesia by activating PPARγ receptors and attenuating neuroinflammatory changes in the striatum.”
Inhibitory effects of cannabidiol on voltage-dependent sodium currents.

“Cannabis sativa contains many related compounds known as phytocannabinoids. The main psychoactive and non-psychoactive compounds are Δ9-tetrahydrocannabidiol (THC) and cannabidiol (CBD), respectively.
Much of the evidence for clinical efficacy of CBD-mediated anti-epileptic effects has been from case reports or smaller surveys. The mechanisms for CBD’s anticonvulsant effects are unclear and likely involve non-cannabinoid receptor pathways.
CBD is reported to modulate several ion channels, including sodium channels (Nav). Evaluating therapeutic mechanisms and safety of CBD demands a richer understanding of its interactions with central nervous system targets. Here, we used voltage-clamp electrophysiology of HEK-293 cells and iPSC neurons to characterize the effects of CBD on Nav channels.
Our results show that CBD inhibits hNav1.1-1.7 currents, with an IC50 of 1.9-3.8 μM, suggesting that this inhibition could occur at therapeutically relevant concentrations. A steep Hill slope of ~3 suggested multiple interactions of CBD with Nav channels. CBD exhibited resting-state blockade, became more potent at depolarized potentials, and also slowed recovery from inactivation, supporting the idea that CBD binding preferentially stabilizes inactivated Nav channel states. We also found that CBD inhibits other voltage-dependent currents from diverse channels, including bacterial homomeric Nav channel (NaChBac) and voltage-gated potassium channel subunit Kv2.1. Lastly, the CBD block of Nav was temperature-dependent, with potency increasing at lower temperatures.
We conclude that CBD’s mode of action likely involves (1) compound partitioning in lipid membranes, which alters membrane fluidity affecting gating, and (2) undetermined direct interactions with sodium and potassium channels, whose combined effects are loss of channel excitability.”
https://www.ncbi.nlm.nih.gov/pubmed/30219789
http://www.jbc.org/content/early/2018/09/14/jbc.RA118.004929

