 “Over the past decade the phenomenon of cannabis as a legitimate form of treatment for pain has overwhelmed the medical community, especially in the field of pain. From a status of a schedule 1 substance having no currently accepted medical use and being considered to have high potential for abuse, its use has mushroomed to over 50,000 legal medical users per year in Israel alone. There appear to be many reasons behind this phenomenon-medical, sociological, and economical. Thus, what is cannabis? An abusive substance or a medication? Should it be incorporated into current biomedical practice, and how should it be administered? Finally, what is the evidence for the beneficial and detrimental effects of cannabis? This article reviews and discusses the current literature regarding the beneficial and the detrimental effects of medical cannabis in the treatment of pain. We further discuss the problems and challenges facing the medical community in this domain and offer a practical approach to deal with these challenges.”
“Over the past decade the phenomenon of cannabis as a legitimate form of treatment for pain has overwhelmed the medical community, especially in the field of pain. From a status of a schedule 1 substance having no currently accepted medical use and being considered to have high potential for abuse, its use has mushroomed to over 50,000 legal medical users per year in Israel alone. There appear to be many reasons behind this phenomenon-medical, sociological, and economical. Thus, what is cannabis? An abusive substance or a medication? Should it be incorporated into current biomedical practice, and how should it be administered? Finally, what is the evidence for the beneficial and detrimental effects of cannabis? This article reviews and discusses the current literature regarding the beneficial and the detrimental effects of medical cannabis in the treatment of pain. We further discuss the problems and challenges facing the medical community in this domain and offer a practical approach to deal with these challenges.”
Monthly Archives: February 2020
Cannabinoid and Terpenoid Doses are Associated with Adult ADHD Status of Medical Cannabis Patients.
 “The aim of this cross-sectional questionnaire-based study was to identify associations between the doses of cannabinoids and terpenes administered, and symptoms of attention deficit hyperactivity disorder (ADHD).
“The aim of this cross-sectional questionnaire-based study was to identify associations between the doses of cannabinoids and terpenes administered, and symptoms of attention deficit hyperactivity disorder (ADHD).
CONCLUSION:
These findings reveal that the higher-dose consumption of medical cannabis (MC) components (phyto-cannabinoids and terpenes) is associated with ADHD medication reduction.
In addition, high dosage of CBN was associated with a lower ASRS score.
However, more studies are needed in order to fully understand if cannabis and its constituents can be used for management of ADHD.”
Cannabis and Cannabinoids in the Treatment of Rheumatic Diseases.
 “Chronic pain is a common complaint among patients, and rheumatic diseases are a common cause for chronic pain. Current pharmacological interventions for chronic pain are not always useful or safe enough for long-term use.
“Chronic pain is a common complaint among patients, and rheumatic diseases are a common cause for chronic pain. Current pharmacological interventions for chronic pain are not always useful or safe enough for long-term use.
Cannabis and cannabinoids are currently being studied due to their potential as analgesics. In this review we will discuss current literature regarding cannabinoids and cannabis as treatment for rheumatic diseases.
Fibromyalgia is a prevalent rheumatic disease that causes diffuse pain, fatigue, and sleep disturbances. Treatment of this syndrome is symptomatic, and it has been suggested that cannabis and cannabinoids could potentially alleviate some of the symptoms associated with fibromyalgia. In this review we cite some of the evidence that supports this claim. However, data on long-term efficacy and safety of cannabinoid and cannabis use are still lacking.
Cannabinoids and cannabis are commonly investigated as analgesic agents, but in recent years more evidence has accumulated on their potential immune-modulatory effect, supported by results in animal models of certain rheumatic diseases. While results that demonstrate the same effect in humans are still lacking, cannabinoids and cannabis remain potential drugs to alleviate the pain associated with rheumatic diseases, as they were shown to be safe and to cause limited adverse effects.”
The Highs and Lows of Cannabis in Cancer Treatment and Bone Marrow Transplantation.
 “In the last decade, we have observed an increased public and scientific interest in the clinical applications of medical cannabis.
“In the last decade, we have observed an increased public and scientific interest in the clinical applications of medical cannabis.
Currently, the application of cannabinoids in cancer patients is mainly due to their analgesic and anti-emetic effects.
The direct effects of phyto-cannabinoids on cancer cells are under intensive research, and the data remain somewhat inconsistent. Although anti-proliferative properties were observed in vitro, conclusive data from animal models and clinical trials are lacking.
Since immunotherapy of malignant diseases and bone marrow transplantation are integral approaches in hemato-oncology, the immuno-modulatory characteristic of cannabinoids is a fundamental aspect for consideration. The effect of cannabinoids on the immune system is presently under investigation, and some evidence for its immuno-regulatory properties has been shown.
In addition, the interaction of cannabinoids and classical cytotoxic agents is a subject for further investigation. Here we discuss the current knowledge of cannabinoid-based treatments in preclinical models and the limited data in oncological patients. Particularly, we address the possible contradiction between the direct anti-tumor and the immune-modulatory effects of cannabinoids.
Better understanding of the mechanism of cannabinoids influence is essential to design therapies that will allow cannabinoids to be incorporated into the clinic.”
Uncovering the hidden antibiotic potential of Cannabis.
 “The spread of antimicrobial resistance continues to be a priority health concern worldwide, necessitating exploration of alternative therapies.
“The spread of antimicrobial resistance continues to be a priority health concern worldwide, necessitating exploration of alternative therapies.
Cannabis sativa has long been known to contain antibacterial cannabinoids, but their potential to address antibiotic resistance has only been superficially investigated.
Here, we show that cannabinoids exhibit antibacterial activity against MRSA, inhibit its ability to form biofilms and eradicate pre-formed biofilms and stationary phase cells persistent to antibiotics.
We show that the mechanism of action of cannabigerol is through targeting the cytoplasmic membrane of Gram-positive bacteria and demonstrate in vivo efficacy of cannabigerol in a murine systemic infection model caused by MRSA.
We also show that cannabinoids are effective against Gram-negative organisms whose outer membrane is permeabilized, where cannabigerol acts on the inner membrane.
Finally, we demonstrate that cannabinoids work in combination with polymyxin B against multi-drug resistant Gram-negative pathogens, revealing the broad-spectrum therapeutic potential for cannabinoids.”
Cannabis Sativa Revisited-Crosstalk between microRNA Expression, Inflammation, Oxidative Stress, and Endocannabinoid Response System in Critically Ill Patients with Sepsis.
 “Critically ill patients with sepsis require a multidisciplinary approach, as this situation implies multiorgan distress, with most of the bodily biochemical and cellular systems being affected by the condition. Moreover, sepsis is characterized by a multitude of biochemical interactions and by dynamic changes of the immune system. At the moment, there is a gap in our understanding of the cellular, genetic, and molecular mechanisms involved in sepsis.
“Critically ill patients with sepsis require a multidisciplinary approach, as this situation implies multiorgan distress, with most of the bodily biochemical and cellular systems being affected by the condition. Moreover, sepsis is characterized by a multitude of biochemical interactions and by dynamic changes of the immune system. At the moment, there is a gap in our understanding of the cellular, genetic, and molecular mechanisms involved in sepsis.
One of the systems intensely studied in recent years is the endocannabinoid signaling pathway, as light was shed over a series of important interactions of cannabinoid receptors with biochemical pathways, specifically for sepsis. Furthermore, a series of important implications on inflammation and the immune system that are induced by the activity of cannabinoid receptors stimulated by the delta-9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) have been noticed.
One of the most important is their ability to reduce the biosynthesis of pro-inflammatory mediators and the modulation of immune mechanisms. Different studies have reported that cannabinoids can reduce oxidative stress at mitochondrial and cellular levels.
The aim of this review paper was to present, in detail, the important mechanisms modulated by the endocannabinoid signaling pathway, as well as of the molecular and cellular links it has with sepsis. At the same time, we wish to present the possible implications of cannabinoids in the most important biological pathways involved in sepsis, such as inflammation, redox activity, immune system, and epigenetic expression.”
https://www.ncbi.nlm.nih.gov/pubmed/32012914
https://www.mdpi.com/2073-4409/9/2/307
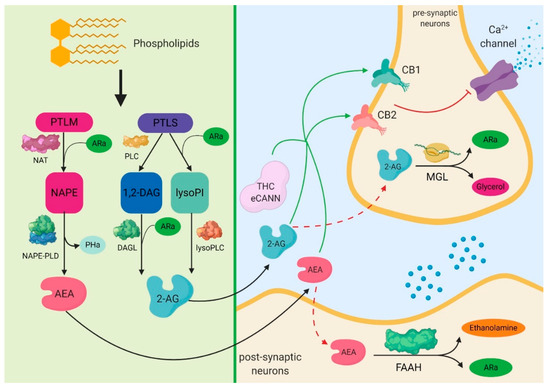
The endocannabinoid receptors CB1 and CB2 affect the regenerative potential of adipose tissue MSCs.
 “Human adipose tissue includes large quantities of mesenchymal stromal cells (atMSCs), which represent an abundant cell source for therapeutic applications in the field of regenerative medicine.
“Human adipose tissue includes large quantities of mesenchymal stromal cells (atMSCs), which represent an abundant cell source for therapeutic applications in the field of regenerative medicine.
Adipose tissue secrets various soluble factors including endocannabinoids, and atMSCs express the cannabinoid receptors CB1 and CB2. This indicates that adipose tissue possesses an endocannabinoid system (ECS). The ECS is also ascribed great significance for wound repair, e.g. by modulating inflammation. However, the exact effects of CB1/CB2 activation in human atMSCs have not been investigated, yet.
In the present study, we stimulated human atMSCs with increasing concentrations (1-30 μM) of the unspecific cannabinoid receptor ligand WIN55,212-2 and the specific CB2 agonist JWH-133, either alone or co-applied with the receptor antagonist Rimonabant (CB1) or AM 630 (CB2). We investigated the effects on metabolic activity, cell number, differentiation and cytokine release, which are important processes during tissue regeneration.
WIN decreased metabolic activity and cell number, which was reversed by Rimonabant. This suggests a CB1 dependent mechanism, whereas the number of atMSCs was increased after CB2 ligation. WIN and JWH increased the release of VEGF, TGF-β1 and HGF. Adipogenesis was enhanced by WIN, which could be reversed by blocking CB1. There was no effect on osteogenesis, and only WIN increased chondrogenic differentiation.
Our results indicate that definite activation of the cannabinoid receptors exerted different effects in atMSCs, which could be of specific value in cell-based therapy for wound regeneration.”
https://www.ncbi.nlm.nih.gov/pubmed/32006556
https://www.sciencedirect.com/science/article/abs/pii/S001448272030080X?via%3Dihub

Organophosphate agent induces ADHD-like behaviors via inhibition of brain endocannabinoid-hydrolyzing enzyme(s) in adolescent male rats.
 “Anticholinergic organophosphate (OP) agents act on the diverse serine hydrolases, thereby revealing unexpected biological effects. Epidemiological studies indicate a relationship between OP exposure and development of attention-deficit/hyperactivity disorder (ADHD)-like symptoms, whereas no plausible mechanism for the OP-induced ADHD has been established.
“Anticholinergic organophosphate (OP) agents act on the diverse serine hydrolases, thereby revealing unexpected biological effects. Epidemiological studies indicate a relationship between OP exposure and development of attention-deficit/hyperactivity disorder (ADHD)-like symptoms, whereas no plausible mechanism for the OP-induced ADHD has been established.
The present investigation employs ethyl octylphosphonofluoridate (EOPF) as an OP-probe which is an extremely potent inhibitor of endocannabinoid (EC, anandamide and 2-arachidonoylglycerol)-hydrolyzing enzymes: i.e., fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL).
Ex vivo experiment shows that EOPF treatment decreases FAAH and MAGL activities and conversely increases EC levels in rat brain. Subsequently, EOPF (treated intraperitoneally once at 0, 1, 2, or 3 mg/kg) clearly induces ADHD-like behaviors (in elevated plus-maze test) in both Wistar and spontaneously hypertensive rats. The EOPF-induced behaviors are reduced by a concomitant administration of cannabinoid receptor inverse agonist SLV-319.
Accordingly, EC system is a feasible target for OP-caused ADHD-like behaviors in adolescent rats.”
Insulinotropic and antidiabetic effects of β-caryophyllene with l-arginine in type 2 diabetic rats.
 “Beta-caryophyllene (BCP) is a flavoring agent, whereas l-arginine (LA) is used as a food supplement.
“Beta-caryophyllene (BCP) is a flavoring agent, whereas l-arginine (LA) is used as a food supplement.
They possess insulinotropic and β cell regeneration activities, respectively.
We assessed the antidiabetic potential of BCP, LA, and its combination in RIN-5F cell lines and diabetic rats.
The results indicated that the combination of BCP with LA showed a significant decrease in glucose absorption and an increase in its uptake in tissues and also an increase in insulin secretion in RIN-5F cells. The combination treatment of BCP with LA showed a significant reduction in glucose, lipid levels, and oxidative stress in pancreatic tissue when compared with the diabetic group. Furthermore, the combination of BCP with LA normalized glucose tolerance and pancreatic cell damage in diabetic rats.
In conclusion, the combinational treatment showed significant potentials in the treatment of type 2 diabetes mellitus.
PRACTICAL APPLICATIONS:
Type 2 diabetes mellitus is the most prevalent chronic metabolic disorder affecting a large population.
Beta-caryophyllene is a CB2 receptor agonist shown to have insulinotropic activity.
l-Arginine is a food supplement that possesses beta-cell regeneration property.
The combination of BCP with LA could work as a potential therapeutic intervention, considering the individual pharmacological activities of each.
We evaluated the antidiabetic activity of the combination of BCP with LA in diabetic rats using ex vivo and in vitro experimentations.
Results from the study revealed that the combination of BCP with LA showed a significant (p < .001) reduction in glucose and lipid levels as compared to individual treatment. In vitro study also supports the diabetic potential of the combination of BCP with LA in the glucose-induced insulin secretion in RIN-5F cell lines.
The study indicates a therapeutic approach to treat T2DM by BCP and LA combination as food and dietary supplement.”
https://www.ncbi.nlm.nih.gov/pubmed/31997410
https://onlinelibrary.wiley.com/doi/abs/10.1111/jfbc.13156
“β-caryophyllene (BCP) is a common constitute of the essential oils of numerous spice, food plants and major component in Cannabis.” http://www.ncbi.nlm.nih.gov/pubmed/23138934
“Beta-caryophyllene is a dietary cannabinoid.” https://www.ncbi.nlm.nih.gov/pubmed/18574142
Activation of CB2R with AM1241 ameliorates neurodegeneration via the Xist/miR-133b-3p/Pitx3 axis.
 “Activation of cannabinoid receptor type II (CB2R) by AM1241 has been demonstrated to protect dopaminergic neurons in Parkinson’s disease (PD) animals.
“Activation of cannabinoid receptor type II (CB2R) by AM1241 has been demonstrated to protect dopaminergic neurons in Parkinson’s disease (PD) animals.
However, the specific mechanisms of the action of the CB2R agonist AM1241 for PD treatment have not been characterized.
The CB2 receptor agonist AM1241 alleviated PD via regulation of the Xist/miR-133b-3p/Pitx3 axis, and revealed a new approach for PD treatment.”
