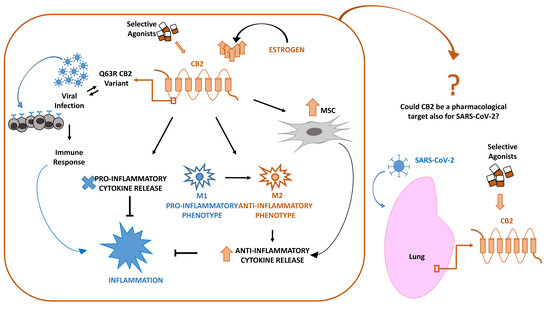
“In late December 2019, a novel coronavirus (SARS-CoV-2 or CoV-19) appeared in Wuhan, China, causing a global pandemic. SARS-CoV-2 causes mild to severe respiratory tract inflammation, often developing into lung fibrosis with thrombosis in pulmonary small vessels and causing even death. COronaVIrus Disease (COVID-19) patients manifest exacerbated inflammatory and immune responses, cytokine storm, prevalence of pro-inflammatory M1 macrophages and increased levels of resident and circulating immune cells. Men show higher susceptibility to SARS-CoV-2 infection than women, likely due to estrogens production. The protective role of estrogens, as well as an immune-suppressive activity that limits the excessive inflammation, can be mediated by cannabinoid receptor type 2 (CB2). The role of this receptor in modulating inflammation and immune response is well documented in fact in several settings. The stimulation of CB2 receptors is known to limit the release of pro-inflammatory cytokines, shift the macrophage phenotype towards the anti-inflammatory M2 type and enhance the immune-modulating properties of mesenchymal stromal cells. For these reasons, we hypothesize that CB2 receptor can be a therapeutic target in COVID-19 pandemic emergency.”
“In late December 2019, a novel coronavirus (SARS-CoV-2 or CoV-19) appeared in Wuhan, China, causing a global pandemic. SARS-CoV-2 causes mild to severe respiratory tract inflammation, often developing into lung fibrosis with thrombosis in pulmonary small vessels and causing even death. COronaVIrus Disease (COVID-19) patients manifest exacerbated inflammatory and immune responses, cytokine storm, prevalence of pro-inflammatory M1 macrophages and increased levels of resident and circulating immune cells. Men show higher susceptibility to SARS-CoV-2 infection than women, likely due to estrogens production. The protective role of estrogens, as well as an immune-suppressive activity that limits the excessive inflammation, can be mediated by cannabinoid receptor type 2 (CB2). The role of this receptor in modulating inflammation and immune response is well documented in fact in several settings. The stimulation of CB2 receptors is known to limit the release of pro-inflammatory cytokines, shift the macrophage phenotype towards the anti-inflammatory M2 type and enhance the immune-modulating properties of mesenchymal stromal cells. For these reasons, we hypothesize that CB2 receptor can be a therapeutic target in COVID-19 pandemic emergency.”
https://pubmed.ncbi.nlm.nih.gov/32471272/
https://www.mdpi.com/1422-0067/21/11/3809


 “Cannabis (Cannabis sativa L.) is a complex, polymorphic plant species, which produces a vast array of bioactive metabolites, the two major chemical groups being cannabinoids and terpenoids. Nonetheless, the psychoactive cannabinoid tetrahydrocannabinol (Δ 9 -THC) and the non-psychoactive cannabidiol (CBD), are the two major cannabinoids that have monopolized the research interest.
“Cannabis (Cannabis sativa L.) is a complex, polymorphic plant species, which produces a vast array of bioactive metabolites, the two major chemical groups being cannabinoids and terpenoids. Nonetheless, the psychoactive cannabinoid tetrahydrocannabinol (Δ 9 -THC) and the non-psychoactive cannabidiol (CBD), are the two major cannabinoids that have monopolized the research interest.
 “Several neuropharmacological actions of cannabidiol (CBD) due to the modulation of the endocannabinoid system as well as direct serotonergic and gamma-aminobutyric acidergic actions have recently been identified.
“Several neuropharmacological actions of cannabidiol (CBD) due to the modulation of the endocannabinoid system as well as direct serotonergic and gamma-aminobutyric acidergic actions have recently been identified. “Four pivotal randomized placebo-controlled trials have demonstrated that adjunctive therapy with cannabidiol (CBD) improves seizure control in patients with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS).
“Four pivotal randomized placebo-controlled trials have demonstrated that adjunctive therapy with cannabidiol (CBD) improves seizure control in patients with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS). “The market for products featuring hemp extracts is large and growing larger. However, safety concerns have been raised by medical and regulatory agencies. Post marketing surveillance of full spectrum hemp extract (FSHE) products manufactured and distributed by CV Sciences (CVSI) and traded under the brand PlusCBD™ was conducted over a 2-year period (2018-2019). The safety of these products was assessed by analyzing adverse events reports.
“The market for products featuring hemp extracts is large and growing larger. However, safety concerns have been raised by medical and regulatory agencies. Post marketing surveillance of full spectrum hemp extract (FSHE) products manufactured and distributed by CV Sciences (CVSI) and traded under the brand PlusCBD™ was conducted over a 2-year period (2018-2019). The safety of these products was assessed by analyzing adverse events reports. “Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.
“Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.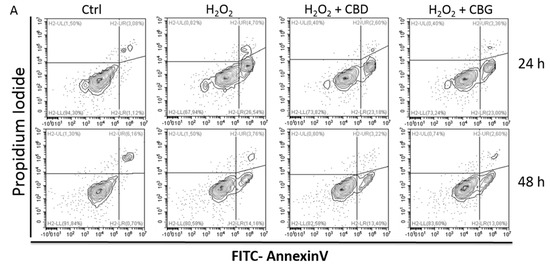
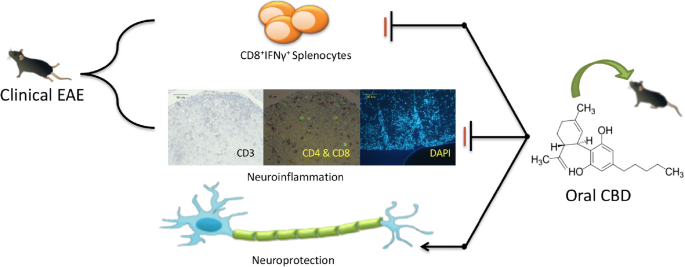
 “In this study cannabidiol (CBD) was administered orally to determine its effects and mechanisms in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). We hypothesized that 75 mg/kg of oral CBD given for 5 days after initiation of disease would reduce EAE severity through suppression of either the early peripheral immune or late neuroimmune response.
“In this study cannabidiol (CBD) was administered orally to determine its effects and mechanisms in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). We hypothesized that 75 mg/kg of oral CBD given for 5 days after initiation of disease would reduce EAE severity through suppression of either the early peripheral immune or late neuroimmune response.