“Cannabinoid receptor 1 (CB1) is a therapeutically relevant drug target for controlling pain, obesity, and other central nervous system disorders. However, full agonists and antagonists of CB1 have been reported to cause serious side effects in patients. Therefore, partial agonists have emerged as a viable alternative as they can mitigate overstimulation and side effects. One of the key bottlenecks in the design of partial agonists, however, is the lack of understanding of the molecular mechanism of partial agonism itself. In this study, we examine two mechanistic hypotheses for the origin of partial agonism in cannabinoid receptors and predict the mechanistic basis of partial agonism exhibited by Δ9-Tetrahydrocannabinol (THC) against CB1. In particular, we inspect whether partial agonism emerges from the ability of THC to bind in both agonist and antagonist-binding poses or from its ability to only partially activate the receptor. We used extensive molecular dynamics simulations and Markov state modeling to capture the THC binding in both antagonist and agonist-binding poses in the CB1 receptor. Furthermore, we predict that binding of THC in the agonist-binding pose leads to rotation of toggle switch residues and causes partial outward movement of intracellular transmembrane helix 6 (TM6). Our simulations also suggest that the alkyl side chain of THC plays a crucial role in determining partial agonism by stabilizing the ligand in the agonist and antagonist-like poses within the pocket. Taken together, this study provides important insights into the mechanistic origin of the partial agonism of THC.”
Monthly Archives: April 2022
Low-Dose Delta-9-Tetrahydrocannabinol as Beneficial Treatment for Aged APP/PS1 Mice
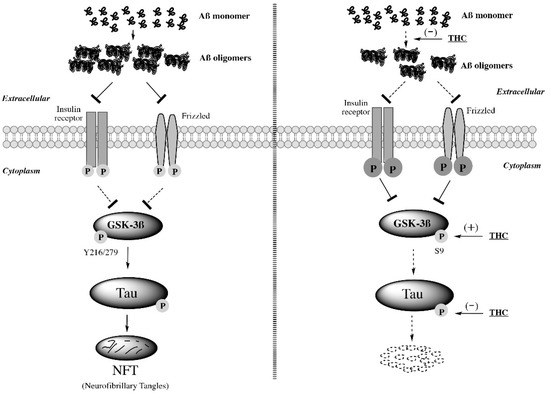
“Studies on the effective and safe therapeutic dosage of delta-9-tetrahydrocannabinol (THC) for the treatment of Alzheimer’s disease (AD) have been sparse due to the concern about THC’s psychotropic activity. The present study focused on demonstrating the beneficial effect of low-dose THC treatment in preclinical AD models.
The effect of THC on amyloid-β (Aβ) production was examined in N2a/AβPPswe cells. An in vivo study was conducted in aged APP/PS1 transgenic mice that received an intraperitoneal injection of THC at 0.02 and 0.2 mg/kg every other day for three months.
The in vitro study showed that THC inhibited Aβ aggregation within a safe dose range. Results of the radial arm water maze (RAWM) test demonstrated that treatment with 0.02 and 0.2 mg/kg of THC for three months significantly improved the spatial learning performance of aged APP/PS1 mice in a dose-dependent manner.
Results of protein analyses revealed that low-dose THC treatment significantly decreased the expression of Aβ oligomers, phospho-tau and total tau, and increased the expression of Aβ monomers and phospho-GSK-3β (Ser9) in the THC-treated brain tissues.
In conclusion, treatment with THC at 0.2 and 0.02 mg/kg improved the spatial learning of aged APP/PS1 mice, suggesting low-dose THC is a safe and effective treatment for AD.”
https://pubmed.ncbi.nlm.nih.gov/35269905/
https://www.mdpi.com/1422-0067/23/5/2757
Thromboembolic Outcomes in Tetrahydrocannabinol-Positive Trauma Patients With Traumatic Brain Injury
“Introduction: Traumatic brain injury (TBI) is a significant source of morbidity and mortality in the United States. Recent shifts in state legislation have increased the use of recreational and medical marijuana. While cannabinoids and tetrahydrocannabinol (THC) have known anti-inflammatory effects, the impact of preinjury THC use on clinical outcomes in the setting of severe TBI is unknown. We hypothesized that preinjury THC use in trauma patients suffering TBI would be associated with decreased thromboembolic events and adverse outcomes.
Methods: The American College of Surgeons Trauma Quality Improvement Program was used to identify patients aged ≥18 y with TBI and severe injury (Injury Severity Score ≥ 16) in admit year 2017. Patients with smoking or tobacco history or missing or positive toxicology tests for drug and/or alcohol use other than THC were excluded. Propensity score matching was used to compare THC+ patients to similar THC- patients.
Results: A total of 13,266 patients met inclusion criteria, of which 1669 were THC+. A total of 1377 THC+ patients were matched to 1377 THC- patients. No significant differences were found in in-hospital outcomes, including mortality, length of stay, cardiac arrest, pulmonary embolism, deep vein thrombosis, or acute respiratory distress syndrome. No patients had ischemic stroke, and THC+ patients had significantly decreased rates of hemorrhagic stroke (0.5% versus 1.5%, P = 0.02, odds ratio 0.41 [95% confidence interval 0.18-0.86]).
Conclusions: Preinjury THC use may be associated with decreased hemorrhagic stroke in severely injured patients with TBI, but there was no difference in thromboembolic outcomes. Further research into pathophysiological mechanisms related to THC are needed.”
https://pubmed.ncbi.nlm.nih.gov/35305485/
“THC linked to lower hemorrhagic stroke risk in people with traumatic brain injury”
Anti-cancer potential of cannabis terpenes in a taxol-resistant model of breast cancer
“Chemotherapeutic resistance can limit breast cancer outcomes; therefore, the exploration of novel therapeutic options is warranted. Isolated compounds found in cannabis have previously been shown to exhibit anti-cancer effects, but little is known about their effects in resistant breast cancer. Our study aims to evaluate the effects of terpenes found in cannabis in in vitro chemotherapy-resistant model of breast cancer. We aimed to identify whether five terpenes found in cannabis produced anti-cancer effects, and if their effects were improved upon co-treatment with cannabinoids and flavonoids also found in cannabis. Nerolidol and β-caryophyllene produced the greatest cytotoxic effects, activated the apoptotic cascade and reduced cellular invasion. Combinations with the flavonoid kaempferol potentiated the cytotoxic effects of ocimene, terpinolene, and β-myrcene. Combinations of nerolidol and Δ9-tetrahydrocannabinol or cannabidiol produced variable responses ranging from antagonism and additivity to synergy, depending on concentrations used. Our results indicate that cannabis terpenes, alone or combined with cannabinoids and flavonoids, produced anti-cancer effects in chemotherapy-resistant breast cancer cell lines. This study is a first step in the identification of compounds that could have therapeutic potential in the treatment of resistant breast cancer.”
https://www.biorxiv.org/content/10.1101/2021.10.08.463667v1.full
The Endocannabinoid System as a Pharmacological Target for New Cancer Therapies
“Despite the long history of cannabinoid use for medicinal and ritual purposes, an endogenous system of cannabinoid-controlled receptors, as well as their ligands and the enzymes that synthesise and degrade them, was only discovered in the 1990s. Since then, the endocannabinoid system has attracted widespread scientific interest regarding new pharmacological targets in cancer treatment among other reasons.
Meanwhile, extensive preclinical studies have shown that cannabinoids have an inhibitory effect on tumour cell proliferation, tumour invasion, metastasis, angiogenesis, chemoresistance and epithelial-mesenchymal transition (EMT) and induce tumour cell apoptosis and autophagy as well as immune response. Appropriate cannabinoid compounds could moreover be useful for cancer patients as potential combination partners with other chemotherapeutic agents to increase their efficacy while reducing unwanted side effects.
In addition to the direct activation of cannabinoid receptors through the exogenous application of corresponding agonists, another strategy is to activate these receptors by increasing the endocannabinoid levels at the corresponding pathological hotspots. Indeed, a number of studies accordingly showed an inhibitory effect of blockers of the endocannabinoid-degrading enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) on tumour development and spread.
This review summarises the relevant preclinical studies with FAAH and MAGL inhibitors compared to studies with cannabinoids and provides an overview of the regulation of the endocannabinoid system in cancer.”
https://pubmed.ncbi.nlm.nih.gov/34830856/
“Cannabinoids have been shown to suppress tumour cell proliferation, tumour invasion, metastasis, angiogenesis, chemoresistance and epithelial-mesenchymal transition and to induce tumour cell apoptosis, autophagy and immune response. This review focuses on the current status of investigations on the impact of inhibitors of endocannabinoid-degrading enzymes on tumour growth and spread in preclinical oncology research.”
https://www.mdpi.com/2072-6694/13/22/5701
Cannabidiol Μay Prolong Survival in Patients With Glioblastoma Multiforme
“Background: Glioblastoma multiforme (GBM) is a relatively rare type of brain tumour with an incidence rate around 6 per 100,000. Even with the widely practiced combination of radiotherapy with adjuvant temozolomide, the median overall survival remains low with just 13.5 to 16 months after diagnosis.
Patients and methods: We retrospectively reviewed the survival of a cohort of 15 consecutive, unselected patients with histopathologically confirmed glioblastoma multiforme (GBM) who received CBD (400 to 600 mg orally per day) in addition to standard therapy (maximum resection of the tumour followed by radio-chemotherapy).
Results: Of 15 patients, seven (46.7%) are now living for at least 24 months, and four (26.7%) for at least 36 months. This is more than twice as long as has been previously reported in the literature. The mean overall survival is currently 24.2 months (median 21 months).
Conclusion: CBD is a well supported co-medication and seems to prolong the survival of patients with glioblastoma multiforme.”
https://pubmed.ncbi.nlm.nih.gov/35403130/
“In conclusion, concomitant CBD seems to prolong the survival of patients with glioblastoma multiforme; CBD was well supported and did not cause side effects.”
Administration of Δ 9-Tetrahydrocannabinol Following Controlled Cortical Impact Restores Hippocampal-Dependent Working Memory and Locomotor Function
“Hypothesis: Administration of the phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC) will enhance brain repair and improve short-term spatial working memory in mice following controlled cortical impact (CCI) by upregulating granulocyte colony-stimulating factor (G-CSF) and other neurotrophic factors (brain-derived neurotrophic factor [BDNF], glial-derived neurotrophic factor [GDNF]) in hippocampus (HP), cerebral cortex, and striatum. Results: Δ9-THC-treated mice exhibited marked improvement in performance on the Y-maze indicating that treatment with the phytocannabinoid could reverse the deficit in working memory caused by the CCI. Δ9-THC-treated mice ran on the rotarod longer than vehicle-treated mice and recovered to normal rotarod performance levels at 2 weeks. Δ9-THC-treated mice, compared with vehicle-treated animals, exhibited significant upregulation of G-CSF as well as BDNF and GDNF in the cerebral cortex, striatum, and HP. Levels of 2-AG were also increased in the Δ9-THC-treated mice. Conclusion: Administration of the phytocannabinoid Δ9-THC promotes significant functional recovery from traumatic brain injury (TBI) in the realms of working memory and locomotor function. This beneficial effect is associated with upregulation of brain 2-AG, G-CSF, BDNF, and GDNF. The latter three neurotrophic factors have been previously shown to mediate brain self-repair following TBI and stroke.”
New AKT-dependent mechanisms of anti-COVID-19 action of high-CBD Cannabis sativa extracts
“COVID-19 is caused by the SARS-CoV-2 virus, which enters target cells via interactions with ACE2 and TMPRSS2. Here, we show AKT serine/threonine kinase-dependent epigenetic control of ACE2 and TMPRSS2 expression by high-cannabidiol (CBD) cannabis extracts and their individual components. CBD alone and extracts #1, #5, #7, and #129 downregulated ACE2 and TMPRSS2 in lung fibroblast WI-38 cells through AKT-mediated inhibition. miR-200c-3p and let-7a-5p were two contributing miRNAs in CBD-mediated suppression of ACE2 and TMPRSS2. CBD and terpene PTWT2.2 profoundly inhibited ACE2 and TMPRSS2 expression, both individually and in combination. Extracts #1, #5, #7, and #169 suppressed COX2 expression and remarkably attenuated TNFα/IFNγ-triggered induction of proinflammatory factors IL-6 and IL-8 by AKT pathway. The most abundant molecules present in extracts #1 and #7 modulated the expression of COX2, IL-6, and IL-8 both individually and in combination. These results reveal that high-CBD cannabis extracts attenuated ACE2 and TMPRSS2 expression and the induction of inflammatory mediators COX2, IL-6, and IL-8 via the AKT pathway, highlighting their potential anti-COVID-19 features.”
Antimicrobial and Antiviral (SARS-CoV-2) Potential of Cannabinoids and Cannabis sativa: A Comprehensive Review
“Antimicrobial resistance has emerged as a global health crisis and, therefore, new drug discovery is a paramount need. Cannabis sativa contains hundreds of chemical constituents produced by secondary metabolism, exerting outstanding antimicrobial, antiviral, and therapeutic properties. This paper comprehensively reviews the antimicrobial and antiviral (particularly against SARS-CoV-2) properties of C. sativa with the potential for new antibiotic drug and/or natural antimicrobial agents for industrial or agricultural use, and their therapeutic potential against the newly emerged coronavirus disease (COVID-19). Cannabis compounds have good potential as drug candidates for new antibiotics, even for some of the WHO’s current priority list of resistant pathogens. Recent studies revealed that cannabinoids seem to have stable conformations with the binding pocket of the Mpro enzyme of SARS-CoV-2, which has a pivotal role in viral replication and transcription. They are found to be suppressive of viral entry and viral activation by downregulating the ACE2 receptor and TMPRSS2 enzymes in the host cellular system. The therapeutic potential of cannabinoids as anti-inflammatory compounds is hypothesized for the treatment of COVID-19. However, more systemic investigations are warranted to establish the best efficacy and their toxic effects, followed by preclinical trials on a large number of participants.”
Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants
“As a complement to vaccines, small-molecule therapeutic agents are needed to treat or prevent infections by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and its variants, which cause COVID-19. Affinity selection-mass spectrometry was used for the discovery of botanical ligands to the SARS-CoV-2 spike protein. Cannabinoid acids from hemp (Cannabis sativa) were found to be allosteric as well as orthosteric ligands with micromolar affinity for the spike protein. In follow-up virus neutralization assays, cannabigerolic acid and cannabidiolic acid prevented infection of human epithelial cells by a pseudovirus expressing the SARS-CoV-2 spike protein and prevented entry of live SARS-CoV-2 into cells. Importantly, cannabigerolic acid and cannabidiolic acid were equally effective against the SARS-CoV-2 alpha variant B.1.1.7 and the beta variant B.1.351. Orally bioavailable and with a long history of safe human use, these cannabinoids, isolated or in hemp extracts, have the potential to prevent as well as treat infection by SARS-CoV-2.”
