“Parkinson’s disease (PD) is a common neurodegenerative disease characterized by a disorder of the dopaminergic system in the midbrain, causing classical PD motor symptoms. The therapeutic effect of cannabidiol (CBD) on PD has been a research frontier in recent years. However, the pathogenesis of PD and the therapeutic mechanism of cannabinoid remain unclear. To further study the causes of PD and the effect of CBD on PD, we exposed the PD transgenic mouse model to CBD and then estimated the motorial and postural coordination through a modified swim test. Afterwards, the mechanism was investigated via the histopathology of substantia nigra and the gut-brain metabolic analysis in the approach of UHPLC-TOF-MS. The results showed that CBD significantly improved motor deficits of PD model and protected the substantia nigra area. The metabolic function of fatty acid biosynthesis, arginine biosynthesis/metabolism, butanoate (ketone body) metabolism, β-alanine metabolism, and pantothenate/CoA biosynthesis was highlighted in the pathological and therapeutic process along the gut-brain axis. In conclusion, CBD could attenuate PD via the neuroprotective effect on the midbrain. The attenuation of the central nervous system in turn improved motor performance of PD, which might be partially induced by the metabolic interaction between the gut-brain. In view of gut-brain metabolomics, the mechanism of PD pathogenesis and the effect of CBD on PD are highly related to the biosynthesis and metabolism of energy and essential substance.”
Monthly Archives: May 2022
A natural product from Cannabis sativa subsp. sativa inhibits homeodomain-interacting protein kinase 2 (HIPK2), attenuating MPP +-induced apoptosis in human neuroblastoma SH-SY5Y cells

“Homeodomain-interacting protein kinase 2 (HIPK2) is a conserved serine/threonine kinase, which regulate transcription, cell differentiation, proliferation and apoptosis. Previous evidences indicated that HIPK2 could be involved in the pathogenesis of neurodegenerative diseases, suggesting as a novel target for Parkinson’s disease (PD) therapeutic development. Herein, gene microarray analysis was performed to verify the key regulatory function of HIPK2 in PD. (Z)-methylp-hydroxycinnamate (ZMHC, 7) with other eighteen compounds were isolated from Cannabis sativa subsp. sativa, growing in Bama Yao Autonomous County, one of the five largest longevity regions of the world. Intriguingly, ZMHC was identified to bind HIPK2 with high affinity through molecular modeling and molecular dynamics (MD) simulations. Moreover, cell morphology, flow cytometry and western blot assay suggested that ZMHC inhibited HIPK2, which attenuated MPP+-induced apoptosis in SH-SY5Y cells. In conclusion, these findings discovered a natural product that inhibited HIPK2, and highlighted that ZMHC could be a potential precursor agent for future PD therapy.”
https://pubmed.ncbi.nlm.nih.gov/28366826/
https://www.sciencedirect.com/science/article/abs/pii/S0045206816303972?via%3Dihub
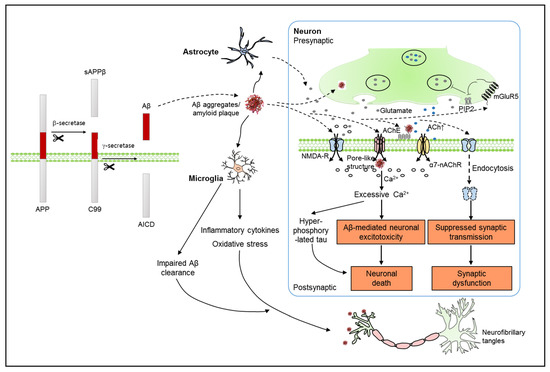
Understanding the Modulatory Effects of Cannabidiol on Alzheimer’s Disease

“Alzheimer’s disease (AD), the most common neurodegenerative disease, is characterized by progressive cognitive impairment. The deposition of amyloid beta (Aβ) and hyperphosphorylated tau is considered the hallmark of AD pathology. Many therapeutic approaches such as Food and Drug Administration-approved cholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists have been used to intervene in AD pathology. However, current therapies only provide limited symptomatic relief and are ineffective in preventing AD progression. Cannabidiol (CBD), a phytocannabinoid devoid of psychoactive responses, provides neuroprotective effects through both cannabinoid and noncannabinoid receptors. Recent studies using an AD mouse model have suggested that CBD can reverse cognitive deficits along with Aβ-induced neuroinflammatory, oxidative responses, and neuronal death. Furthermore, CBD can reduce the accumulation of Aβ and hyperphosphorylation of tau, suggesting the possibility of delaying AD progression. Particularly, the noncannabinoid receptor, peroxisome proliferator-activated receptor gamma, has been suggested to be involved in multiple functions of CBD. Therefore, understanding the underlying mechanisms of CBD is necessary for intervening in AD pathology in depth and for the translation of preclinical studies into clinical settings. In this review, we summarize recent studies on the effect of CBD in AD and suggest problems to be overcome for the therapeutic use of CBD.”
https://pubmed.ncbi.nlm.nih.gov/34573232/
https://www.mdpi.com/2076-3425/11/9/1211
Cannabidiol regulates CB1-pSTAT3 signaling for neurite outgrowth, prolongs lifespan, and improves health span in Caenorhabditis elegans of Aβ pathology models

“Cannabidiol (CBD), a phytocannabinoid from the Cannabis sativa plant, exhibits a broad spectrum of potential therapeutic properties for neurodegenerative diseases. An accumulation of amyloid-β (Aβ) protein is one of the most important neuropathology in neurodegenerative diseases like Alzheimer’s disease (AD). Data on the effect of CBD on the amelioration of Aβ-induced neurite degeneration and its consequences of life and health spans is sparse. This study aimed to investigate the effects of CBD on neurite outgrowth in cells and lifespan and health span in Caenorhabditis elegans (C. elegans). In human SH-SY5Y neuronal cells, CBD prevented neurite lesion induced by Aβ1-42 and increased the expression of fatty acid amide hydrolase (FAAH) and cannabinoid receptor 1 (CB1R). Furthermore, CBD both protected the reduction of dendritic spine density and rescued the activity of synaptic Ca2+ /calmodulin-dependent protein kinase II (CaMKII) from Aβ1-42 toxicity in primary hippocampal neurons. In C. elegans, we used the transgenic CL2355 strain of C. elegans, which expresses the human Aβ peptide throughout the nervous system and found that CBD treatment extended lifespan and improved health span. The neuroprotective effect of CBD was further explored by observing the dopaminergic neurons using transgenic dat-1: GFP strains using the confocal microscope. This study shows that CBD prevents the neurite degeneration induced by Aβ, by a mechanism involving CB1R activation, and extends lifespan and improves health span in Aβ-overexpressing worms. Our findings support the potential therapeutic approach of CBD for the treatment of AD patients.”
https://pubmed.ncbi.nlm.nih.gov/33817834/
“We showed that CBD extends lifespan and improves health span in Aβ-expression C. elegans. Taken together, our findings highlight the neuroprotective benefits of CBD and its therapeutic potential for neurodegenerative conditions such as AD.”
https://faseb.onlinelibrary.wiley.com/doi/10.1096/fj.202002724R
Role and Function of Endocannabinoid System in Major Depressive Disease
“The endocannabinoid system (ECS) is a neuromodulator system with a crucial role in CNS and the reaction to endogenous and exogenous compounds and inflammation. Cannabidiol (CBD) is a basic part of the ECS which is the overwhelming causative and/or protective factor of major depressive disease (MDD). CBD interacts with brain-derived neurotropic factor (BDNF) that responds to inflammation, dysregulations of the hypothalamic-pituitary-adrenal (HPA) axis, and many more imbalances in MDD patients for which the ECS is a vital part to analyze, diagnose, and reflect the treatment. The ECS and MDD appear to have strong connections and interactions, so interest in ECS and CBD use in MDD patients is developing as a rescue resort.”
Cannabinol modulates neuroprotection and intraocular pressure: A potential multi-target therapeutic intervention for glaucoma

“Objectives: Glaucoma is characterized by progressive damage of the retinal ganglion cells (RGCs), resulting in irreversible vision loss. Cannabinoids (CBs) ameliorate several factors that contribute to the progression of glaucoma, including increased intraocular pressure (IOP), degeneration of RGC and optical nerve (ON) damage. However, a direct correlation of specific CBs with the molecular events pertaining to glaucoma pathology is not well established. Therefore, this study aims to evaluate the role of cannabinol (CBN) on RGC protection, modulation of IOP, and its effects on the level of extracellular matrix (ECM) proteins using both in vitro and in vivo models of glaucoma.
Methods and results: When exposed to elevated hydrostatic pressure, CBN, in a dose-dependent manner, protected differentiated mouse 661W retinal ganglion precursor-like cells from pressure-induced toxicity. In human trabecular meshwork cells (hTM), CBN attenuated changes in the ECM proteins, including fibronectin and α-smooth muscle actin (α-SMA), as well as mitogen-activated protein kinases (phospho-ERK1/2) in the presence or absence of transforming growth factor-beta 2 (TGF-β2) induced stress. Ocular pharmacokinetic parameters were evaluated post-intravitreal (IVT) CBN delivery in vivo. Furthermore, we demonstrated that IVT-administered CBN improved pattern electroretinogram (pERG) amplitudes and reduced IOP in a rat episcleral vein laser photocoagulation model of glaucoma.
Conclusion: CBN promotes neuroprotection, abrogates changes in ECM protein, and normalizes the IOP levels in the eye. Therefore, our observations in the present study indicate a therapeutic potential for CBN in the treatment of glaucoma.”
https://pubmed.ncbi.nlm.nih.gov/34921975/
“These results suggest CBN as a potential therapeutic intervention for patients with glaucoma.”
https://www.sciencedirect.com/science/article/abs/pii/S0925443921002581?via%3Dihub
Protective effect of cannabinoids on gastric mucosal lesions induced by water immersion restrain stress in rats
“Objectives: This study aimed to determine the impact of cannabinoid agonists and antagonists on the mucosal lesion progress in the stomach induced by water-immersion restraint stress (WIRS).
Materials and methods: Rats subjected to WIRS for 4 hr were treated with Dimethyl sulfoxide (DMSO), CBR1 agonist (NADA, 1 mg/kg), CBR1 antagonist (Rimonabant, 1 mg/kg), CBR2 agonist (GW405833 1 mg/kg) or CBR2 antagonist (AM630, 1 mg/kg SC) 30 min before WIRS. Microscopic lesions, oxidative stress, inflammatory cytokines biomarkers, and (Myeloperoxidase) MPO in gastric tissues were determined.
Results: Results indicated development of severe gastric lesions with a substantial increase in the contents of (nitric oxide) NO, (malondialdehyde) MDA, (interleukin-1 beta) IL-1β, MPO, (tumor necrosis factor-alpha) TNF-α, and a significant fall in the content of GSH and the activity of PON-1 after WIRS.
Conclusion: Treatment with NADA and AM630 protected gastric tissues against ulcers as demonstrated by a decrease in the contents of MDA, TNF-α, MPO, and IL-1β along with an increase in the content of PON-1 activity and GSH in the stomach tissues. On the other hand, treatment with SR141716A or GW405833 showed no protective effects on ulcers development. It seems that cannabinoids exert their antioxidant potential and anti-inflammatory effects against WIRS-induced gastric ulcers by activation of CB1R.”
Cannabidiol protects against Alzheimer’s disease in C. elegans via ROS scavenging activity of its phenolic hydroxyl groups

“Recent discoveries have implicated the potential of Cannabidiol (CBD) in the prevention of Alzheimer’s disease (AD). However, how CBD affects such neurodegenerative disorders remains unclear. Herein, Caenorhabditis elegans (C. elegans) was used as the model organism to elucidate the mechanism by which CBD ameliorates AD in vivo. CBD was found to alleviate the progression of Aβ-induced AD but not tau protein-induced AD or α-syn-induced Parkinson’s disease. CBD inhibited the aggregation of Aβ in C. elegans. However, CBD failed to prevent the formation of β-sheet aggregation in vitro. Moreover, CBD was found to scavenge reactive oxygen species (ROS) in vivo without inducing the overexpression of antioxidative genes. In addition, CBD treatment enhanced the worm resistance to oxidative stress, which was independent of the classical transcription factors DAF-16 and SKN-1. These results supported that the in vivo antioxidative activity of CBD was most likely due to its intrinsic antioxidative property. Furthermore, the phenolic hydroxyl groups of CBD were found to be critical for scavenging ROS in vitro and in vivo, alleviating the aggregation of Aβ in vivo, and ameliorating Aβ-associated neurotoxicity. These studies show that CBD protects against AD in C. elegans via the ROS scavenging activity of its phenolic hydroxyl groups, which provides insight for further structure-activity relationship studies of CBD as an AD therapeutic.”
https://pubmed.ncbi.nlm.nih.gov/35181336/
https://www.sciencedirect.com/science/article/abs/pii/S0014299922000905?via%3Dihub
Neuroprotection of retinal ganglion cells in vivo using the activation of the endogenous cannabinoid signaling system in mammalian eyes

“Cannabinoid and glutamatergic signaling systems in the human retina coexist and greatly influence one another. Under glaucomatous conditions, excess levels of glutamate accrete in the retinal ganglion cell (RGC) layer. The present study tests the putative neuroprotective effect mediated by cannabinoids at the CB1 and CB2 receptors. In the first experiment, mice were given intravitreal injections of 160 nmol N-methyl-d-aspartic acid (NMDA) in one eye and saline in the paired eye. In the second experiment, both eyes were given NMDA, while one of the two was additionally given the cannabinoid agonist WIN 55,212-2. Ten days later, animals were perfused and the retinae were dissected as wholemounts and stained with Cresyl Violet. Quantitative analysis revealed that 70% of the neurons in the retinal ganglion cell (RGC) layer exposed to NMDA underwent cell death. The addition of the cannabinoid CB1/CB2 agonist doubled the number of neurons surviving the NMDA treatment. These data provide evidence that cannabinoids, either exogenous or endogenous, may be harnessed to provide protection from neurodegenerative diseases, including glaucoma, and from glutamate-induced, and potentially other forms of neurotoxicity, under chronic or acute conditions.”
https://pubmed.ncbi.nlm.nih.gov/35233292/
“In summary, we have demonstrated that the cannabimimetic drug, the CB1 and CB2 receptor agonist WIN55,212-2, acts to protect RGCs from NMDA-induced excitotoxicity in an in vivo mouse model. This further indicates the potential for therapeutic applications of cannabinoids in neurodegenerative diseases, including glaucoma.”
Cannabinol Inhibits Cellular Proliferation, Invasion, and Angiogenesis of Neuroblastoma via Novel miR-34a/tRiMetF31/PFKFB3 Axis

“High-risk neuroblastoma is an aggressive pediatric tumor. Despite great advances in neuroblastoma therapy and supportive care protocols, no curative treatment is available for most patients with this disease. Here, we uncover that CBN attenuated the cell proliferation, invasion, and angiogenesis of neuroblastoma cell lines in a dose-dependent manner via the inhibition of the AKT pathway and the upregulation of miR-34a that targets E2F1. Both miR-34a and a 31-nt tRNAiMet fragment (tRiMetF31) derived from miR-34a-guided cleavage were downregulated in 4 examined neuroblastoma cell lines inversely correlated with the levels of its direct target, the PFKFB3 protein. Moreover, ectopic tRiMetF31 suppressed proliferation, migration, and angiogenesis in the studied neuroblastoma cell lines. Conversely, tRiMetF31 knockdown promoted PFKFB3 expression, resulting in enhanced angiogenesis. Our findings reveal a suppressive role of CBN in neuroblastoma tumorigenesis, highlighting a novel and crucial miR-34a tumor suppressor network in CBN’s antineuroblastoma actions.”
https://pubmed.ncbi.nlm.nih.gov/35454815/
“Cannabinol is a chemical found in the Cannabis sativa plant.”
https://www.webmd.com/vitamins/ai/ingredientmono-1611/cannabinol-cbn
