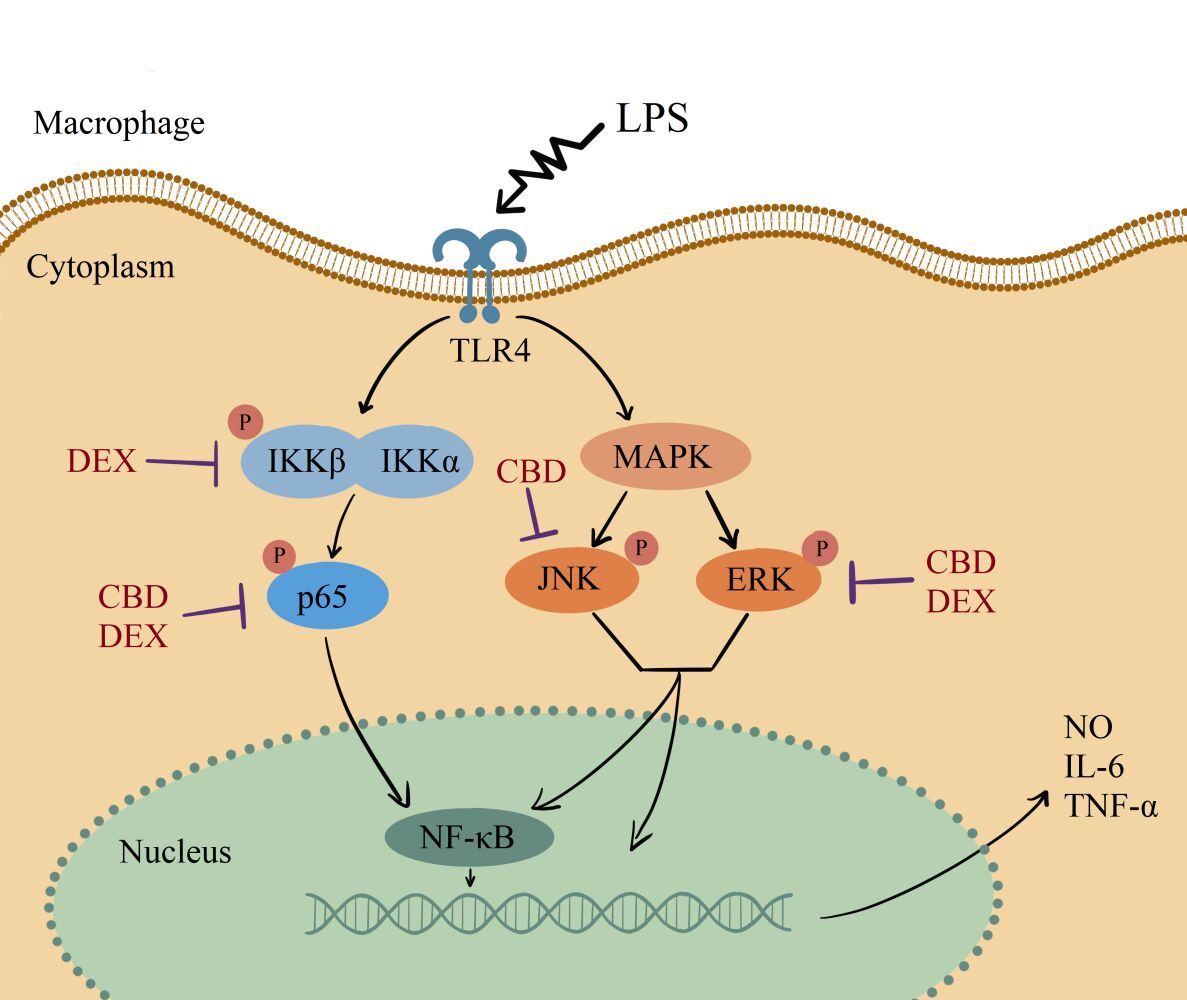
“Dermatological diseases pose a significant burden on the quality of life of individuals and can be challenging to treat effectively. In this aspect, cannabinoids are gaining increasing importance due to their therapeutic potential in various disease entities including skin diseases. In this synthetic review, we comprehensively analyzed the existing literature in the field of potential dermatological applications of a lesser-known subgroup of cannabinoids, the so-called minor cannabinoids, such as cannabidivarin (CBDV), cannabidiforol (CBDP), cannabichromene (CBC), tetrahydrocannabivarin (THCV), cannabigerolic acid (CBGA), cannabigerol (CBG), cannabielsoin (CBE), cannabimovone (CBM) or cannabinol (CBN), while drawing attention to their unique pharmacological properties. We systematically searched the available databases for relevant studies and analyzed the data to provide an overview of current thematic knowledge. We looked through the full-text, bibliographic and factographic databases, especially Scopus, Web of Science, PubMed, Polish Scientific Journals Database, and selected the most relevant papers. Our review highlights that minor cannabinoids exhibit diverse pharmacological activities, including anti-inflammatory, analgesic, antimicrobial, and anti-itch properties. Several studies have reported their efficacy in mitigating symptoms associated with dermatological diseases such as psoriasis, eczema, acne, and pruritus. Furthermore, minor cannabinoids have shown potential in regulating sebum production, a crucial factor in acne pathogenesis. The findings of this review suggest that minor cannabinoids hold therapeutic promise in the management of dermatological diseases. Further preclinical and clinical investigations are warranted to elucidate their mechanisms of action, determine optimal dosage regimens, and assess long-term safety profiles. Incorporating minor cannabinoids into dermatological therapies could potentially offer novel treatment options of patients and improve their overall well-being.”