
“Cannabis-based terpenes are believed to modulate physiological responses to disease and alter the efficacy of cannabinoids in the so-called “entourage effect”. The monoterpene myrcene can reduce nociception produced by noxious thermal and mechanical stimuli as well as reducing acute inflammation.
The current study examined the role of myrcene and cannabidiol (CBD) in controlling chronic joint inflammation and pain.
Chronic arthritis was induced in male Wistar rats by intra-articular injection of Freund’s complete adjuvant into the right knee. On days 7 and 21 after arthritis induction, joint pain (von Frey hair algesiometry), inflammation (intravital microscopy, laser speckle contrast analysis) and joint histopathology were assessed.
Local application of myrcene (1 and 5 mg/kg s.c.) reduced joint pain and inflammation via a cannabinoid receptor mechanism. The combination of myrcene and CBD (200 μg) was not significantly different from myrcene alone. Repeated myrcene treatment had no effect on joint damage or inflammatory cytokine production.
These data suggest that topical myrcene has the potential to reduce chronic arthritis pain and inflammation; however, it has no synergistic effect with CBD.”
https://pubmed.ncbi.nlm.nih.gov/35887239/
“In summary, myrcene was found to have anti-inflammatory and analgesic effects in inflammatory joint disease by activating articular cannabinoid receptors. Together, these findings may explain why arthritis patients prefer Cannabis strains rich in myrcene to help manage their pain and inflammation.”


 “The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.  “In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite.
“In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite. 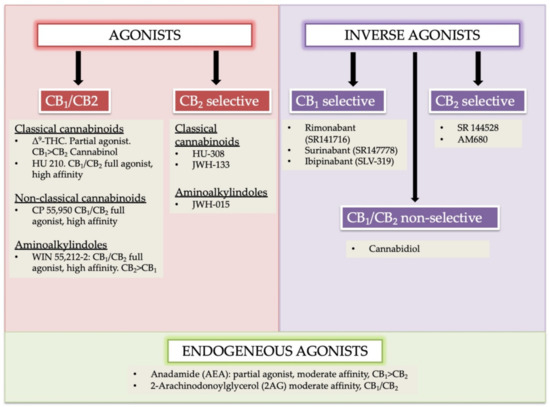
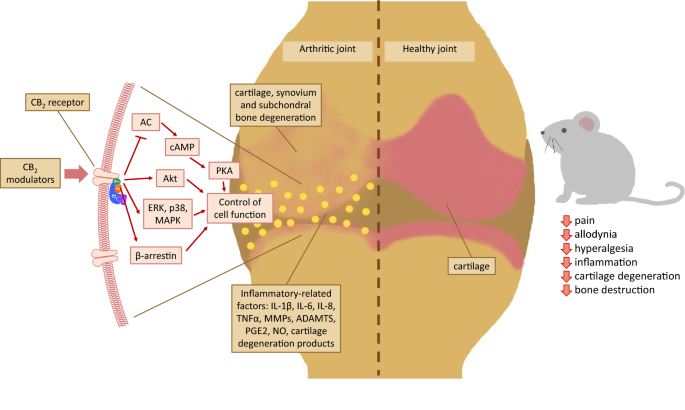 “Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50-60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility.
“Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50-60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility.  “Cannabis sativa L. is an aromatic annual herb belonging to the family Cannabaceae and it is widely distributed worldwide. Cultivation, selling, and consumption of cannabis and cannabis related products, regardless of its use, was prohibited in Lebanon until April 22, 2020. Nevertheless, cannabis oil has been traditionally used unlawfully for many years in Lebanon to treat diseases such as arthritis, diabetes, cancer and few neurological disorders.
“Cannabis sativa L. is an aromatic annual herb belonging to the family Cannabaceae and it is widely distributed worldwide. Cultivation, selling, and consumption of cannabis and cannabis related products, regardless of its use, was prohibited in Lebanon until April 22, 2020. Nevertheless, cannabis oil has been traditionally used unlawfully for many years in Lebanon to treat diseases such as arthritis, diabetes, cancer and few neurological disorders.
 “Background/objectives: Use of cannabis is increasing in a variety of populations in the United States; however, few investigations about how and for what reasons cannabis is used in older populations exist.
“Background/objectives: Use of cannabis is increasing in a variety of populations in the United States; however, few investigations about how and for what reasons cannabis is used in older populations exist.