 “Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.
“Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.
Objectives: To clarify the role of delta-9-tetrahydrocannabinol(THC):cannabidiol(CBD) oromucosal spray, coupled to robot-aided gait training (RAGT) using the Lokomat©Pro to improve functional ambulation in patients with MS.
Methods: We compared 20 patients with MS, who were treated with THC:CBD oromucosal spray in add-on to the ongoing oral antispastic therapy (OAT) (group A), with 20 individuals with MS (matched for clinical-demographic characteristics) who were treated only with OAT (group B). Both the groups underwent RAGT using the Lokomat-Pro (three 45-minute sessions per week). Our primary outcome measures were the Functional Independence Measure (FIM) and the 10 meters walking test (10MWT). As secondary outcome measures we evaluated the brain cortical excitability by using Transcranial Magnetic Stimulation. Both parameters were taken before and after the end of the RAGT.
Results: FIM improved in group A more than in group B (p<0.001). Moreover, 10MWT decreased in group A more than in group B (p<0.001). These clinical findings were paralleled by a more evident reshape of intracortical excitability in both upper and lower limbs, as suggested by motor evoked potential amplitude increase (p<0.001), intracortical inhibition strengthening (p<0.001), and intracortical facilitation decrease (p=0.01) in group A as compared to group B.
Conclusions: Our results suggest that the combined THC:CBD-RAGT approach could be useful in improving gait performance in patients with MS.”
https://pubmed.ncbi.nlm.nih.gov/32447249/
“The coupled therapy is preliminarily demonstrated as safe and efficacious.”
https://www.msard-journal.com/article/S2211-0348(20)30253-4/pdf
 “Psoriasis is associated with increased production of reactive oxygen species which leads to oxidative stress.
“Psoriasis is associated with increased production of reactive oxygen species which leads to oxidative stress.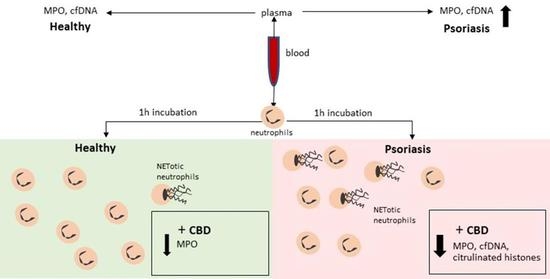

 “The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator.
“The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator. “HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy.
“HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy.
 “Painful tonic spasm (PTS) is a common yet debilitating symptom in patients with neuromyelitis optica spectrum disorder (NMOSD), especially those with longitudinally extensive transverse myelitis. Although carbamazepine is an effective treatment, it poses the risk of severe adverse reactions, such as Steven-Johnson syndrome (SJS).
“Painful tonic spasm (PTS) is a common yet debilitating symptom in patients with neuromyelitis optica spectrum disorder (NMOSD), especially those with longitudinally extensive transverse myelitis. Although carbamazepine is an effective treatment, it poses the risk of severe adverse reactions, such as Steven-Johnson syndrome (SJS). “Moderate to severe spasticity is commonly reported in Multiple Sclerosis (MS) and its management is still a challenge. Cannabinoids were recently suggested as add-on therapy for the treatment of spasticity and chronic pain in MS but there is no conclusive scientific evidence on their safety, especially on cognition and over long periods.
“Moderate to severe spasticity is commonly reported in Multiple Sclerosis (MS) and its management is still a challenge. Cannabinoids were recently suggested as add-on therapy for the treatment of spasticity and chronic pain in MS but there is no conclusive scientific evidence on their safety, especially on cognition and over long periods. “Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.
“Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain. “The approval of 9-δ-tetrahydocannabinol (THC)+
“The approval of 9-δ-tetrahydocannabinol (THC)+ “Cannabidiol (CBD) has been shown by our laboratory to attenuate experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS).
“Cannabidiol (CBD) has been shown by our laboratory to attenuate experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). “The endocannabinoid system (ECS) is an extensive endogenous signaling system with multiple elements, the number of which may be increasing as scientists continue to elucidate its role in human health and disease. The ECS is seemingly ubiquitous in animal species and is modulated by diet, sleep, exercise, stress, and a multitude of other factors, including exposure to phytocannabinoids, like
“The endocannabinoid system (ECS) is an extensive endogenous signaling system with multiple elements, the number of which may be increasing as scientists continue to elucidate its role in human health and disease. The ECS is seemingly ubiquitous in animal species and is modulated by diet, sleep, exercise, stress, and a multitude of other factors, including exposure to phytocannabinoids, like