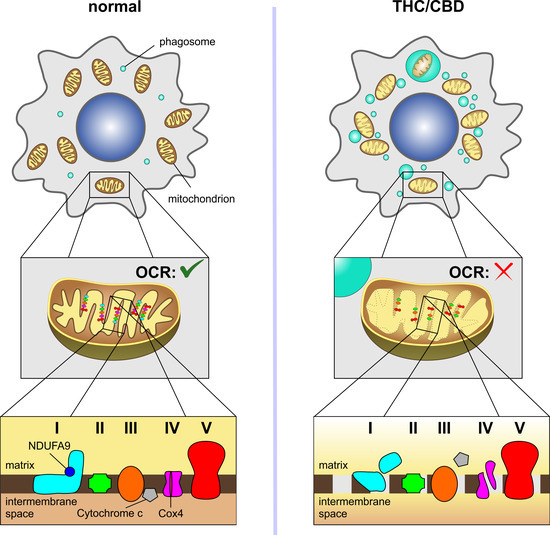
“Melanoma is one of the deadliest forms of cancer. Most melanoma deaths are caused by distant metastases in several organs, especially the brain, the so-called melanoma brain metastases (MBMs). However, the precise mechanisms that sustain the growth of MBMs remain elusive. Recently, the excitatory neurotransmitter glutamate has been proposed as a brain-specific, pro-tumorigenic signal for various types of cancers, but how neuronal glutamate shuttling onto metastases is regulated remains unknown. Here, we show that the cannabinoid CB1 receptor (CB1R), a master regulator of glutamate output from nerve terminals, controls MBM proliferation. First, in silico transcriptomic analysis of cancer-genome atlases indicated an aberrant expression of glutamate receptors in human metastatic melanoma samples. Second, in vitro experiments conducted on three different melanoma cell lines showed that the selective blockade of glutamatergic NMDA receptors, but not AMPA or metabotropic receptors, reduces cell proliferation. Third, in vivo grafting of melanoma cells in the brain of mice selectively devoid of CB1Rs in glutamatergic neurons increased tumour cell proliferation in concert with NMDA receptor activation, whereas melanoma cell growth in other tissue locations was not affected. Taken together, our findings demonstrate an unprecedented regulatory role of neuronal CB1Rs in the MBM tumour microenvironment.”