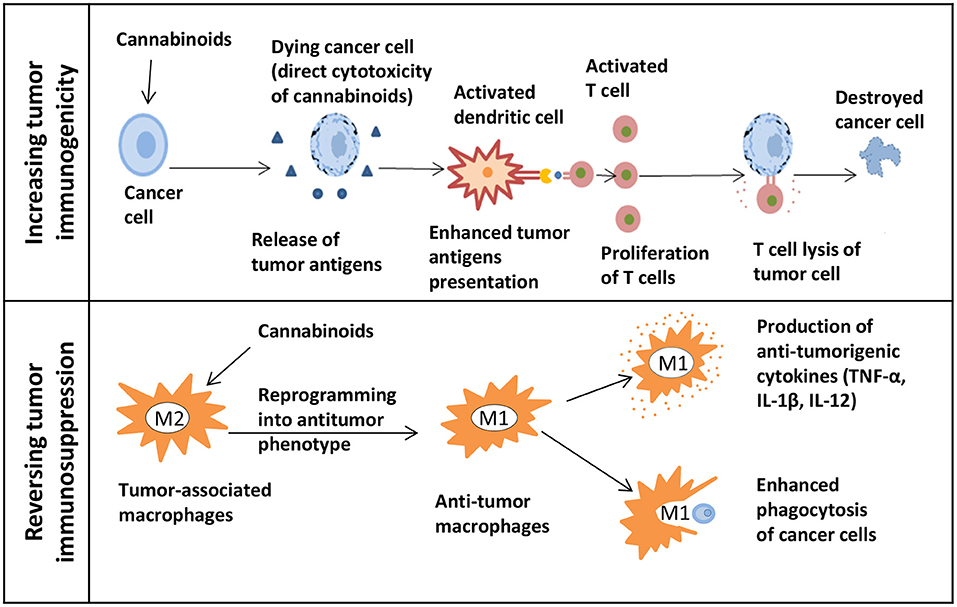
“Plant-based, synthetic, and endogenous cannabinoids have been shown to control a diverse array of biological processes, including regulation of cell fate across cancers. Their promise as broad-based antitumor agents in preclinical models has led to the initiation of pilot clinical trials. Session 5 of the National Cancer Institute’s Cannabis, Cannabinoids and Cancer Research Symposium provides an overview of this research topic.
Overall, the presentations highlight cannabinoid signal transduction and specific molecular mechanisms underlying cannabinoid antitumor activity. They also demonstrate the broad-based antitumor activity of the plant-based, synthetic, and endogenous cannabinoid compounds. Importantly, evidence is presented demonstrating when cannabinoids may be contraindicated as a treatment for cancer, as in the case of human papilloma virus-meditated oropharynx cancer or potentially other p38 MAPK pathway-driven cancers.
Finally, it is discussed that a key to advancing cannabinoids into the clinic is to conduct well-designed, large-scale clinical trials to determine whether cannabinoids are effective antitumor agents in cancer patients.”
https://pubmed.ncbi.nlm.nih.gov/34850900/
“These sessions present multiple lines of preclinical evidence supporting that the cannabinoids THC and CBD act as broad-based antitumor agents controlling many aspects of cancer progression, including cell proliferation, apoptosis, invasion, metastasis, and immune surveillance. “
https://academic.oup.com/jncimono/article/2021/58/99/6446216?login=false