 “Cannabinoids are a group of terpenophenolic compounds derived from the Cannabis sativa L. plant. There is a growing body of evidence from cell culture and animal studies in support of cannabinoids possessing anticancer properties.
“Cannabinoids are a group of terpenophenolic compounds derived from the Cannabis sativa L. plant. There is a growing body of evidence from cell culture and animal studies in support of cannabinoids possessing anticancer properties.
Method: A database search of peer reviewed articles published in English as full texts between January 1970 and April 2021 in Google Scholar, MEDLINE, PubMed and Web of Science was undertaken. References of relevant literature were searched to identify additional studies to construct a narrative literature review of oncological effects of cannabinoids in pre-clinical and clinical studies in various cancer types.
Results: Phyto-, endogenous and synthetic cannabinoids demonstrated antitumour effects both in vitro and in vivo. However, these effects are dependent on cancer type, the concentration and preparation of the cannabinoid and the abundance of receptor targets. The mechanism of action of synthetic cannabinoids, (-)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) has mainly been described via the traditional cannabinoid receptors; CB1 and CB2, but reports have also indicated evidence of activity through GPR55, TRPM8 and other ion channels including TRPA1, TRPV1 and TRPV2.
Conclusion: Cannabinoids have shown to be efficacious both as a single agent and in combination with antineoplastic drugs. These effects have occurred through various receptors and ligands and modulation of signalling pathways involved in hallmarks of cancer pathology. There is a need for further studies to characterise its mode of action at the molecular level and to delineate efficacious dosage and route of administration in addition to synergistic regimes.”
https://pubmed.ncbi.nlm.nih.gov/34259916/
“Since time immemorial, the Cannabis plant has been used as a source of fibre, herbal remedy, medicinal and religious purposes. In the mid-nineteenth century, O’Shaughnessy and Moreau reported positive effects of cannabis on muscle spasms, vomiting, convulsions, rheumatism, tetanus, and rabies. However, during the twentieth century, its utilisation in Western medicine started to decline as a result of political prejudices and economic interests rather than scientific or medical reasons.
Plant-based, endogenous and synthetic cannabinoid compounds have shown merits in not only alleviating the unwanted side effects of antineoplastic drug regiments, but have also shown promising evidence in decreasing tumour burden, and one in vivo study so far concludes increasing survival rates in mice. Various extracted forms of cannabinoids from C. sativa have shown varying cytotoxic effects which should be explored in more detail in future studies as majority of the evidence originates from studies investigating mainly ∆9-THC and CBD’s actions.”
https://link.springer.com/article/10.1007/s00432-021-03710-7
“Introduction: Cannabidiol (CBD) can be isolated from Cannabis sativa L. or synthetically produced. The aim of this study was to compare the in vitro effects of purified natural and synthetic CBD to establish any pharmacological differences or superiority between sources.

 “Extracellular Vesicles (EVs) were isolated from human umbilical cord mesenchymal stem cells (hUCMSCs) and were further encapsulated with cannabidiol (CBD) through sonication method (CBD EVs). CBD EVs displayed an average particle size of 114.1±1.02 nm, zeta potential of -30.26±0.12 mV, entrapment efficiency of 92.3±2.21% and stability for several months at 4 °C. CBD release from the EVs was observed as 50.74±2.44% and 53.99±1.4% at pH 6.8 and pH 7.4, respectively after 48 h. Ourin-vitrostudies demonstrated that CBD either alone or in EVs form significantly sensitized MDA-MB-231 cells to doxorubicin (DOX) (*P<0.05). Flow cytometry and migration studies revealed that CBD EVs either alone or in combination with DOX induced G1 phase cell cycle arrest and decreased migration of MDA-MB-231 cells, respectively. CBD EVs and DOX combination significantly reduced tumor burden (***P<0.001) in MDA-MB-231 xenograft tumor model. Western blotting and immunocytochemical analysis demonstrated that CBD EVs and DOX combination decreased the expression of proteins involved in inflammation, metastasis and increased the expression of proteins involved in apoptosis. CBD EVs and DOX combination will have profound clinical significance in not only decreasing the side effects but also increasing the therapeutic efficacy of DOX in TNBC.”
“Extracellular Vesicles (EVs) were isolated from human umbilical cord mesenchymal stem cells (hUCMSCs) and were further encapsulated with cannabidiol (CBD) through sonication method (CBD EVs). CBD EVs displayed an average particle size of 114.1±1.02 nm, zeta potential of -30.26±0.12 mV, entrapment efficiency of 92.3±2.21% and stability for several months at 4 °C. CBD release from the EVs was observed as 50.74±2.44% and 53.99±1.4% at pH 6.8 and pH 7.4, respectively after 48 h. Ourin-vitrostudies demonstrated that CBD either alone or in EVs form significantly sensitized MDA-MB-231 cells to doxorubicin (DOX) (*P<0.05). Flow cytometry and migration studies revealed that CBD EVs either alone or in combination with DOX induced G1 phase cell cycle arrest and decreased migration of MDA-MB-231 cells, respectively. CBD EVs and DOX combination significantly reduced tumor burden (***P<0.001) in MDA-MB-231 xenograft tumor model. Western blotting and immunocytochemical analysis demonstrated that CBD EVs and DOX combination decreased the expression of proteins involved in inflammation, metastasis and increased the expression of proteins involved in apoptosis. CBD EVs and DOX combination will have profound clinical significance in not only decreasing the side effects but also increasing the therapeutic efficacy of DOX in TNBC.” “Our laboratory is interested in searching for a new plant-based therapeutics to treat ovarian cancer.
“Our laboratory is interested in searching for a new plant-based therapeutics to treat ovarian cancer. “In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite.
“In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite. 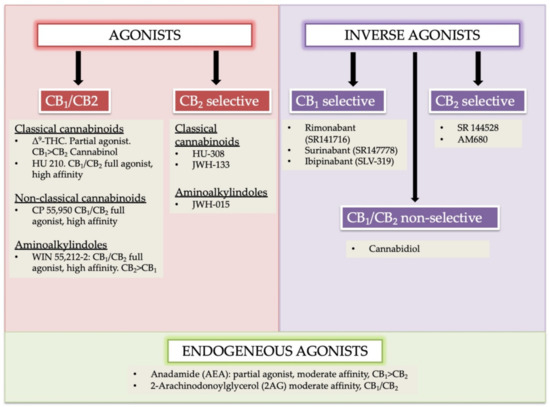
 “Melanoma is one of the most aggressive malignances in human. Recently developed therapies improved overall survival rate, however, the treatment of melanoma still remains a challenging issue.
“Melanoma is one of the most aggressive malignances in human. Recently developed therapies improved overall survival rate, however, the treatment of melanoma still remains a challenging issue. 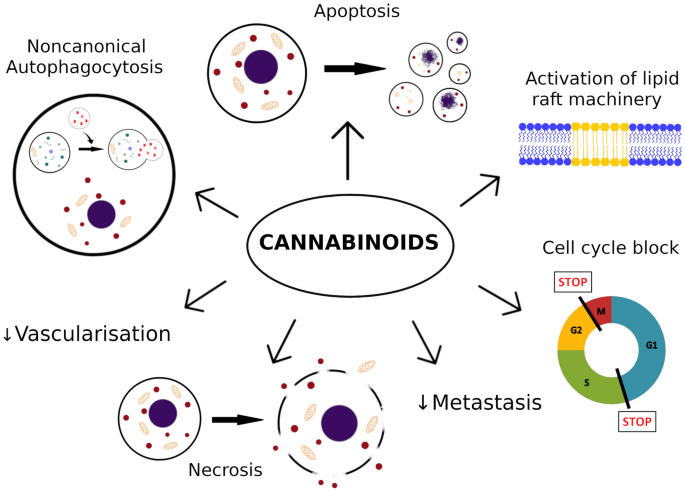
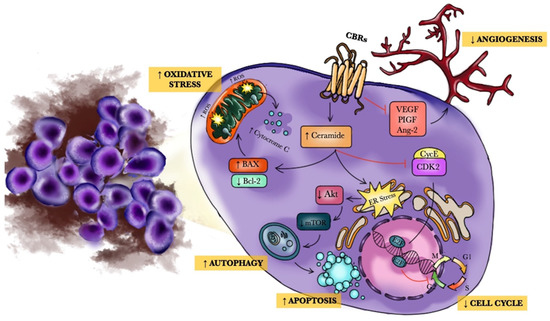
 “Medical marijuana (MM) use is common among cancer patients, but relatively little is known about the usage patterns and efficacy of MM used by gynecologic cancer patients.
“Medical marijuana (MM) use is common among cancer patients, but relatively little is known about the usage patterns and efficacy of MM used by gynecologic cancer patients. “This study aimed to obtain and characterize extracted hemp oil enriched in cannabidiol (CBD) by decarboxylation of cannabidiolic acid (CBDA) and to give new insights into its antioxidant and anticancer effects.
“This study aimed to obtain and characterize extracted hemp oil enriched in cannabidiol (CBD) by decarboxylation of cannabidiolic acid (CBDA) and to give new insights into its antioxidant and anticancer effects.