“Several studies support, both in vitro and in vivo, the anti-cancer effects of cannabidiol (CBD), a transient receptor potential vanilloid 2 (TRPV2) ligand. TRPV2, often dysregulated in tumors, is associated with altered cell proliferation and aggressiveness.
“Several studies support, both in vitro and in vivo, the anti-cancer effects of cannabidiol (CBD), a transient receptor potential vanilloid 2 (TRPV2) ligand. TRPV2, often dysregulated in tumors, is associated with altered cell proliferation and aggressiveness.
Endometrial cancer (EC) is historically divided in type I endometrioid EC and type II non-endometrioid EC, associated with poor prognosis. Treatment options with chemotherapy and combinations with radiation showed only limited efficacy. Since no data are reported concerning TRPV2 expression as well as CBD potential effects in EC, the aim of this study was to evaluate the expression of TRPV2 in biopsies and cell lines as well as the effects of CBD in in vitro models. Overall survival (OS), progression-free survival (PFS), cell viability, migration, and chemo-resistance have been evaluated.
Results show that TRPV2 expression increased with the malignancy of the cancer tissue and correlated with shorter PFS (p = 0.0224). Moreover, in vitro TRPV2 over-expression in Ishikawa cell line increased migratory ability and response to cisplatin. CBD reduced cell viability, activating predominantly apoptosis in type I cells and autophagy in mixed type EC cells. The CBD improved chemotherapeutic drugs cytotoxic effects, enhanced by TRPV2 over-expression. Hence, TRPV2 could be considered as a marker for optimizing the therapy and CBD might be a useful therapeutic option as adjuvant therapy.”
https://pubmed.ncbi.nlm.nih.gov/32751388/
https://www.mdpi.com/1422-0067/21/15/5409


 “In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”
“In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”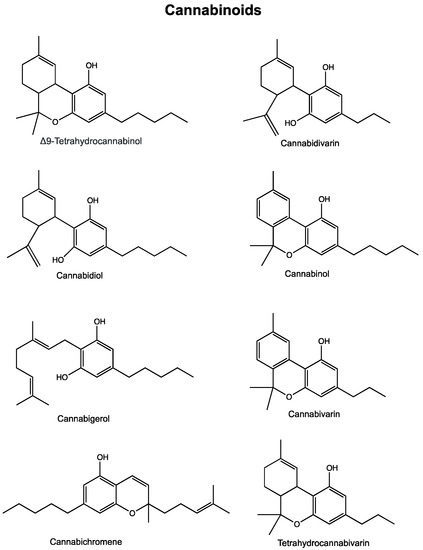
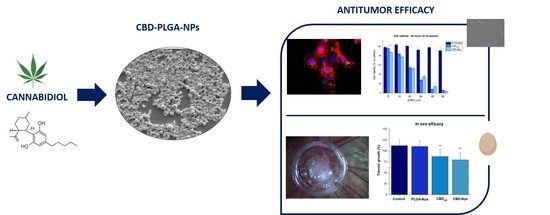
 “In this work, we evaluated, for the first time, the antitumor effect of cannabidiol (CBD) as monotherapy and in combination with conventional chemotherapeutics in ovarian cancer and developed PLGA-microparticles as CBD carriers to optimize its anticancer activity.
“In this work, we evaluated, for the first time, the antitumor effect of cannabidiol (CBD) as monotherapy and in combination with conventional chemotherapeutics in ovarian cancer and developed PLGA-microparticles as CBD carriers to optimize its anticancer activity.


 “Astrocytomas, the most prevalent primary brain tumors, can be divided by histology and malignancy levels into four following types: pilocytic astrocytoma (grade I), diffuse fibrillary astrocytoma (grade II), anaplastic astrocytoma (grade III), and glioblastoma multiforme (grade IV). For high grade astrocytomas (grade III and grade IV), blood vessels formation is considered as the most important property.
“Astrocytomas, the most prevalent primary brain tumors, can be divided by histology and malignancy levels into four following types: pilocytic astrocytoma (grade I), diffuse fibrillary astrocytoma (grade II), anaplastic astrocytoma (grade III), and glioblastoma multiforme (grade IV). For high grade astrocytomas (grade III and grade IV), blood vessels formation is considered as the most important property.
 ‘T-cell acute lymphoblastic leukemia (T-ALL) is a highly heterogeneous malignant hematological disorder arising from T-cell progenitors.
‘T-cell acute lymphoblastic leukemia (T-ALL) is a highly heterogeneous malignant hematological disorder arising from T-cell progenitors. “The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival.
“The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival. “The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
“The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.