 “Cannabinoids exhibit anti-inflammatory and antitumorigenic properties.
“Cannabinoids exhibit anti-inflammatory and antitumorigenic properties.
Contrary to most cannabinoids present in the Cannabis plant, some, such as O-1602 and abnormal cannabidiol, have no or only little affinity to the CB1 or CB2 cannabinoid receptors and instead exert their effects through other receptors.
Here, we investigated whether the synthetic regioisomers of cannabidiol, abnormal cannabidiol, and a closely related compound, O-1602, display antitumorigenic effects in cellular models of breast cancer and whether it could reduce tumorigenesis in vivo.
Several studies have shown the effects of cannabinoids on chemotherapy-sensitive breast cancer cell lines, but less is known about the antitumorigenic effects of cannabinoids in chemotherapy-resistant cell lines.
Paclitaxel-resistant MDA-MB-231 and MCF-7 breast cancer cell lines were used to study the effect of O-1602 and abnormal cannabidiol on viability, apoptosis, and migration. The effects of O-1602 and abnormal cannabidiol on cell viability were completely blocked by the combination of GPR55 and GPR18-specific siRNAs. Both O-1602 and abnormal cannabidiol decreased viability in paclitaxel-resistant breast cancer cells in a concentration-dependent manner through induction of apoptosis. The effect of these cannabinoids on tumor growth in vivo was studied in a zebrafish xenograft model. In this model, treatment with O-1602 and abnormal cannabidiol (2 µM) significantly reduced tumor growth.
Our results suggest that atypical cannabinoids, like O-1602 and abnormal cannabidiol, exert antitumorigenic effects on paclitaxel-resistant breast cancer cells. Due to their lack of central sedation and psychoactive effects, these atypical cannabinoids could represent new leads for the development of additional anticancer treatments when resistance to conventional chemotherapy occurs during the treatment of breast and possibly other cancers.”
https://www.ncbi.nlm.nih.gov/pubmed/31611800
“Our results suggest that some cannabinoids acting through the GPR55 and/or GPR18 receptors can be helpful in inducing apoptosis in breast cancer cell lines that are unresponsive to paclitaxel. The effects of O-1602 and Abn-CBD on cell viability were observed both in vitro and in a zebrafish xenograft model. These drugs were also reducing cell migration. Taken together, even if no synergistic antitumor effect is always observed when cannabinoids and chemotherapeutic agents are combined as an anticancer treatment, cannabinoids can still provide anticancer benefits on top of their palliative effects. This is particularly important in the context of cancers that have developed resistance to current chemotherapies.”
https://www.frontiersin.org/articles/10.3389/fphar.2019.01124/full

 “Anticancer properties of non-psychoactive cannabinoid
“Anticancer properties of non-psychoactive cannabinoid  “Cancer-related cachexia and anorexia syndrome (CACS) is a common phenomenon in cancer patients. Cannabis has been suggested to stimulate appetite but research on this issue has yielded mixed results. The current study aimed to evaluate the effect of dosage-controlled cannabis capsules on CACS in advanced cancer patients.
“Cancer-related cachexia and anorexia syndrome (CACS) is a common phenomenon in cancer patients. Cannabis has been suggested to stimulate appetite but research on this issue has yielded mixed results. The current study aimed to evaluate the effect of dosage-controlled cannabis capsules on CACS in advanced cancer patients.
 “Grade IV glioblastoma multiforme is a deadly disease, with a median survival of around 14 to 16 months. Maximal resection followed by adjuvant radiochemotherapy has been the mainstay of treatment since many years, although survival is only extended by a few months. In recent years, an increasing number of data from in vitro and in vivo research with cannabinoids, particularly with the non-intoxicating cannabidiol (CBD), point to their potential role as tumour-inhibiting agents. Herein, a total of nine consecutive patients with brain tumours are described as case series; all patients received CBD in a daily dose of 400 mg concomitantly to the standard therapeutic procedure of maximal resection followed by radiochemotherapy. By the time of the submission of this article, all but one patient are still alive with a mean survival time of 22.3 months (range=7-47 months). This is longer than what would have been expected.”
“Grade IV glioblastoma multiforme is a deadly disease, with a median survival of around 14 to 16 months. Maximal resection followed by adjuvant radiochemotherapy has been the mainstay of treatment since many years, although survival is only extended by a few months. In recent years, an increasing number of data from in vitro and in vivo research with cannabinoids, particularly with the non-intoxicating cannabidiol (CBD), point to their potential role as tumour-inhibiting agents. Herein, a total of nine consecutive patients with brain tumours are described as case series; all patients received CBD in a daily dose of 400 mg concomitantly to the standard therapeutic procedure of maximal resection followed by radiochemotherapy. By the time of the submission of this article, all but one patient are still alive with a mean survival time of 22.3 months (range=7-47 months). This is longer than what would have been expected.”
 “Little is known about the endocannabinoid (eCB) system in squamous cell carcinoma of the oral tongue (SCCOT). Here we have investigated, at the mRNA level, expression of genes coding for the components of the eCB system in tumour and non-malignant samples from SCCOT patients. Expression of NAPEPLD and PLA2G4E, coding for eCB anabolic enzymes, was higher in the tumour tissue than in non-malignant tissue. Among genes coding for eCB catabolic enzymes, expression of MGLL was lower in tumour tissue while PTGS2 was increased. It is concluded that the eCB system may be dysfunctional in SCCOT.”
“Little is known about the endocannabinoid (eCB) system in squamous cell carcinoma of the oral tongue (SCCOT). Here we have investigated, at the mRNA level, expression of genes coding for the components of the eCB system in tumour and non-malignant samples from SCCOT patients. Expression of NAPEPLD and PLA2G4E, coding for eCB anabolic enzymes, was higher in the tumour tissue than in non-malignant tissue. Among genes coding for eCB catabolic enzymes, expression of MGLL was lower in tumour tissue while PTGS2 was increased. It is concluded that the eCB system may be dysfunctional in SCCOT.” “The endogenous lipid metabolism network is associated with the occurrence and progression of malignancies.
“The endogenous lipid metabolism network is associated with the occurrence and progression of malignancies. “Indisputably, cancer is a global crisis that requires immediate intervention. Despite the use of conventional treatments over the past decades, it is acceptable to admit that these are expensive, invasive, associated with many side effects and, therefore, a reduced quality of life.
“Indisputably, cancer is a global crisis that requires immediate intervention. Despite the use of conventional treatments over the past decades, it is acceptable to admit that these are expensive, invasive, associated with many side effects and, therefore, a reduced quality of life.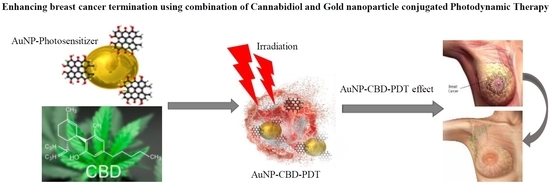
 “Cancer patients experience multiple symptoms throughout their illness, and some report benefit from the use of cannabis. There are concerns that many patients are accessing products inappropriate for their situation and potentially putting themselves at risk.
“Cancer patients experience multiple symptoms throughout their illness, and some report benefit from the use of cannabis. There are concerns that many patients are accessing products inappropriate for their situation and potentially putting themselves at risk.