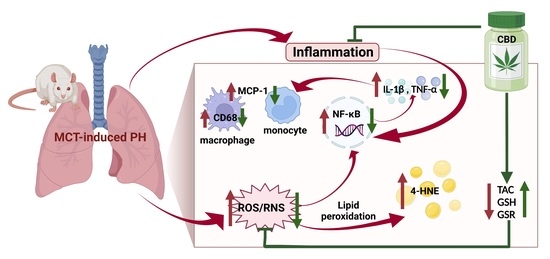
“Background: Sepsis, caused by a dysregulated host response to infections, can lead to cardiac arrhythmias. However, the mechanisms underlying sepsis-induced inflammation, and how inflammation provokes cardiac arrhythmias, are not well understood. We hypothesized that CBD may ameliorate lipopolysaccharides (LPS)-induced cardiotoxicity via Toll-like receptor 4 (TLR-4) and cardiac sodium channels (Nav1.5).
Methods and results: We incubated human immune cells (THP-1 macrophages) with LPS for 24 hours, then extracted the THP-1 incubation media. ELISA assay showed that LPS (1 or 5 μg/ml), in a concentration-dependent manner, or MPLA (TLR-4 agonist, 5 μg/ml) stimulated the THP-1 cells to release inflammatory cytokines (TNF-α and IL-6). Prior incubation (4 hours) with cannabidiol (CBD: 5 μM) or C34 (TLR-4 antagonist: 5 μg/ml) inhibited LPS and MPLA-induced release of both IL-6 and TNF-α. Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) were subsequently incubated for 24 hours in the media extracted from THP-1 cells incubated with LPS, MPLA alone, or in combination with CBD or C34. Voltage-clamp experiments showed a right shift in the voltage dependence of Nav1.5 activation, steady state fast inactivation (SSFI), increased persistent current and prolonged in silico action potential duration in hiSPC-CM incubated in the LPS or MPLA-THP-1 media. Co-incubation with CBD or C34 rescued the biophysical dysfunction caused by LPS and MPLA.
Conclusion: Our results suggest that CBD may protect against sepsis-induced inflammation and subsequent arrhythmias through (i) inhibition of the release of inflammatory cytokines, antioxidant and anti-apoptotic effects and/or (ii) direct effect on Nav1.5.”
https://pubmed.ncbi.nlm.nih.gov/35906756/
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.15936