
 “The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
In addition to regulating physiological processes, the ECS directly influences anxiety, feeding behaviour/appetite, emotional behaviour, depression, nervous functions, neurogenesis, neuroprotection, reward, cognition, learning, memory, pain sensation, fertility, pregnancy, and pre-and post-natal development.
The ECS is also involved in several pathophysiological diseases such as cancer, cardiovascular diseases, and neurodegenerative diseases. In recent years, genetic and pharmacological manipulation of the ECS has gained significant interest in medicine, research, and drug discovery and development.
The distribution of the components of the ECS system throughout the body, and the physiological/pathophysiological role of the ECS-signalling pathways in many diseases, all offer promising opportunities for the development of novel cannabinergic, cannabimimetic, and cannabinoid-based therapeutic drugs that genetically or pharmacologically modulate the ECS via inhibition of metabolic pathways and/or agonism or antagonism of the receptors of the ECS. This modulation results in the differential expression/activity of the components of the ECS that may be beneficial in the treatment of a number of diseases.
This manuscript in-depth review will investigate the potential of the ECS in the treatment of various diseases, and to put forth the suggestion that many of these secondary metabolites of Cannabis sativa L. (hereafter referred to as “C. sativa L.” or “medical cannabis”), may also have potential as lead compounds in the development of cannabinoid-based pharmaceuticals for a variety of diseases.”
https://www.mdpi.com/1422-0067/22/17/9472

 “Objectives:
“Objectives:  “Introduction:
“Introduction: “Objectives: This article presents findings from a large prospective examination of Canadian medical cannabis patients, with a focus on the impacts of cannabis on prescription opioid use and quality of life over a 6-month period.
“Objectives: This article presents findings from a large prospective examination of Canadian medical cannabis patients, with a focus on the impacts of cannabis on prescription opioid use and quality of life over a 6-month period.
 “Although studied in a few randomized controlled trials (RCTs), the efficacy of medical cannabis (MC) for chronic pain remains controversial. Using an alternative approach, this multicenter, questionnaire-based prospective cohort was aimed to assess the long-term effects of MC on chronic pain of various etiologies and to identify predictors for MC treatment success.
“Although studied in a few randomized controlled trials (RCTs), the efficacy of medical cannabis (MC) for chronic pain remains controversial. Using an alternative approach, this multicenter, questionnaire-based prospective cohort was aimed to assess the long-term effects of MC on chronic pain of various etiologies and to identify predictors for MC treatment success.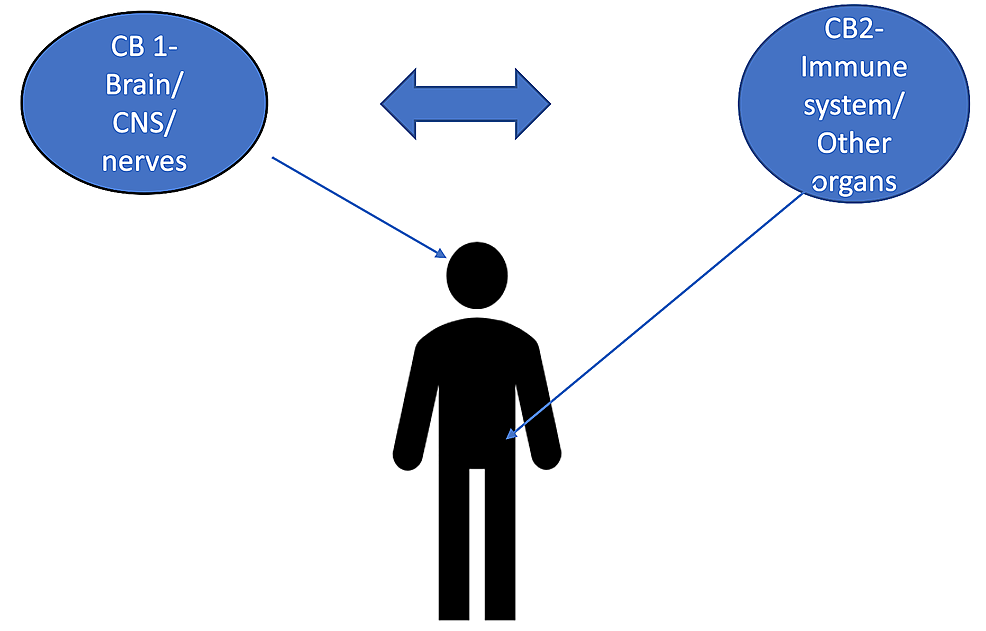 “The burden of chronic pain has affected many individuals leading to distress and discomfort, alongside numerous side effects with conventional therapeutic approaches.
“The burden of chronic pain has affected many individuals leading to distress and discomfort, alongside numerous side effects with conventional therapeutic approaches. “Cannabinoids help in pain treatment through their action on CB1 and CB2 receptors.
“Cannabinoids help in pain treatment through their action on CB1 and CB2 receptors. “Recent years have seen an increase in the adoption of cannabinoid medicines, which have demonstrated effectiveness for the treatment of chronic pain.
“Recent years have seen an increase in the adoption of cannabinoid medicines, which have demonstrated effectiveness for the treatment of chronic pain. “Recent studies have shown that cannabidiol (CBD) could have a great therapeutic potential for treating disorders such as chronic pain and anxiety. In the target article, the authors propose that CBD modulates serotonergic transmission and reverses allodynia and anxiety-like behaviour in a rat model of neuropathic pain. Furthermore, this study shows an antinociceptive effect mediated by TRPV1 and partially by 5-HT1A receptors, as well as an anxiolytic effect mediated by 5-HT1A receptors.”
“Recent studies have shown that cannabidiol (CBD) could have a great therapeutic potential for treating disorders such as chronic pain and anxiety. In the target article, the authors propose that CBD modulates serotonergic transmission and reverses allodynia and anxiety-like behaviour in a rat model of neuropathic pain. Furthermore, this study shows an antinociceptive effect mediated by TRPV1 and partially by 5-HT1A receptors, as well as an anxiolytic effect mediated by 5-HT1A receptors.”