 “Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
“Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
Cannabinoids exert their effects through the endocannabinoid system (ECS) that comprises cannabinoid receptors (CB1, CB2), endogenous ligands, and metabolizing enzymes. Several preclinical studies have demonstrated the potential of cannabinoids against leukemia, lymphoma, glioblastoma, and cancers of the breast, colorectum, pancreas, cervix and prostate.
Cannabis and its constituents can modulate multiple cancer related pathways such as PKB, AMPK, CAMKK-β, mTOR, PDHK, HIF-1α, and PPAR-γ. Cannabinoids can block cell growth, progression of cell cycle and induce apoptosis selectively in tumour cells. Cannabinoids can also enhance the efficacy of cancer therapeutics. These compounds have been used for the management of anorexia, queasiness, and pain in cancer patients.
Cannabinoid based products such as dronabinol, nabilone, nabiximols, and epidyolex are now approved for medical use in cancer patients. Cannabinoids are reported to produce a favourable safety profile. However, psychoactive properties and poor bioavailability limit the use of some cannabinoids. The Academic Institutions across the globe are offering training courses on cannabis. How cannabis and its constituents exert anticancer activities is discussed in this article. We also discuss areas that require attention and more extensive research.”
https://pubmed.ncbi.nlm.nih.gov/33246167/
https://www.sciencedirect.com/science/article/abs/pii/S1043661820316108?via%3Dihub

 “Providers need to be better equipped to discuss medical cannabis with patients even if they are not willing to prescribe it. The oncology community would be well served to ensure that providers are aware of existing cannabis research and are able to incorporate it into their communications with patients instead of leaving patients to figure out medical cannabis on their own.”
“Providers need to be better equipped to discuss medical cannabis with patients even if they are not willing to prescribe it. The oncology community would be well served to ensure that providers are aware of existing cannabis research and are able to incorporate it into their communications with patients instead of leaving patients to figure out medical cannabis on their own.”

 “Multidrug resistance (MDR) to known chemotherapeutic agents is increasing while the development of new drugs is lacking behind. Combination therapies might increase the development of effective treatment.
“Multidrug resistance (MDR) to known chemotherapeutic agents is increasing while the development of new drugs is lacking behind. Combination therapies might increase the development of effective treatment.

 “In this study, we investigated the effects of exposition to IC50 dose for 24 h of a new synthetic cannabinoid (CB83) and of phytocannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on HT-29 colorectal carcinoma cells. Cell viability and proliferative activity evaluated using the MTT, lactate dehydrogenase (LDH), and CyQUANT assays showed that cell viability was significantly affected when CB83, THC, and CBD were administered to cells.
“In this study, we investigated the effects of exposition to IC50 dose for 24 h of a new synthetic cannabinoid (CB83) and of phytocannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on HT-29 colorectal carcinoma cells. Cell viability and proliferative activity evaluated using the MTT, lactate dehydrogenase (LDH), and CyQUANT assays showed that cell viability was significantly affected when CB83, THC, and CBD were administered to cells.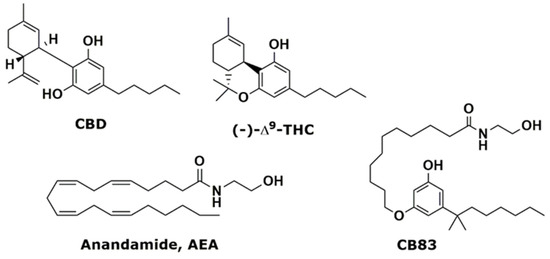
 “In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”
“In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”
 “Colorectal cancer (CRC) has a high mortality rate and is one of the most difficult diseases to manage due to tumour resistance and metastasis. The treatment of choice for CRC is reliant on the phase and time of diagnosis. Despite several conventional treatments available to treat CRC (surgical excision, chemo-, radiation- and immune-therapy), resistance is a major challenge, especially if it has metastasized. Additionally, these treatments often cause unwanted adverse side effects and so it remains imperative to investigate, alternative combination therapies.
“Colorectal cancer (CRC) has a high mortality rate and is one of the most difficult diseases to manage due to tumour resistance and metastasis. The treatment of choice for CRC is reliant on the phase and time of diagnosis. Despite several conventional treatments available to treat CRC (surgical excision, chemo-, radiation- and immune-therapy), resistance is a major challenge, especially if it has metastasized. Additionally, these treatments often cause unwanted adverse side effects and so it remains imperative to investigate, alternative combination therapies. “The recent announcement of marijuana legalization in Canada spiked many discussions about potential health benefits of Cannabis sativa.
“The recent announcement of marijuana legalization in Canada spiked many discussions about potential health benefits of Cannabis sativa.