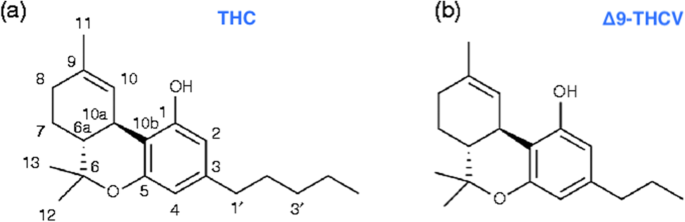
 “Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects. In rodent studies, THCV decreases appetite, increases satiety, and up-regulates energy metabolism, making it a clinically useful remedy for weight loss and management of obesity and type 2 diabetic patients. The distinctions between THCV and THC in terms of glycemic control, glucose metabolism, and energy regulation have been demonstrated in previous studies. Also, the effect of THCV on dyslipidemia and glycemic control in type 2 diabetics showed reduced fasting plasma glucose concentration when compared to a placebo group. In contrast, THC is indicated in individuals with cachexia. However, the uniquely diverse properties of THCV provide neuroprotection, appetite suppression, glycemic control, and reduced side effects, etc.; therefore, making it a potential priority candidate for the development of clinically useful therapies in the future. Hopefully, THCV could provide an optional platform for the treatment of life-threatening diseases.”
“Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects. In rodent studies, THCV decreases appetite, increases satiety, and up-regulates energy metabolism, making it a clinically useful remedy for weight loss and management of obesity and type 2 diabetic patients. The distinctions between THCV and THC in terms of glycemic control, glucose metabolism, and energy regulation have been demonstrated in previous studies. Also, the effect of THCV on dyslipidemia and glycemic control in type 2 diabetics showed reduced fasting plasma glucose concentration when compared to a placebo group. In contrast, THC is indicated in individuals with cachexia. However, the uniquely diverse properties of THCV provide neuroprotection, appetite suppression, glycemic control, and reduced side effects, etc.; therefore, making it a potential priority candidate for the development of clinically useful therapies in the future. Hopefully, THCV could provide an optional platform for the treatment of life-threatening diseases.”
“The psychoactive effects of THC in marijuana are the main reasons for its classification as a Schedule I substance, even though it is the THC that the U.S. Food and Drug Administration (FDA) approved for appetite stimulation and weight gain. In contrast to THC, clinical and therapeutic advantages of THCV regarding its lack of psychoactive effects in human studies are of great value in pharmacotherapy. It is envisioned that the unique and diverse characteristics of THCV could be explored for further development into clinically useful medicines for the treatment of life-threatening diseases.”
https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-020-0016-7

 “In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite.
“In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite. 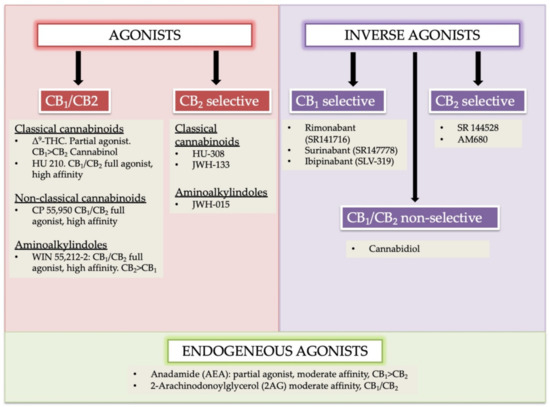
 “(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants.
“(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants. “The endocannabinoid system (ECS) is natural physiological system in the humans. The presence of the ECS system involves different roles in body. The endocannabinoid system involves regulation of most of the centers, which regulates the hunger and leads to changes in the weight.
“The endocannabinoid system (ECS) is natural physiological system in the humans. The presence of the ECS system involves different roles in body. The endocannabinoid system involves regulation of most of the centers, which regulates the hunger and leads to changes in the weight.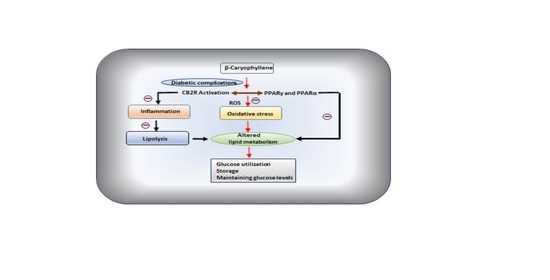
 “While activation of cannabinoid (CB2) receptors has been shown to be neuroprotective, no studies have examined whether this neuroprotection is directed at cerebral arterioles and no studies have examined whether activation of CB2 receptors can rescue cerebrovascular dysfunction during a chronic disease state such as type 1 diabetes (T1D).
“While activation of cannabinoid (CB2) receptors has been shown to be neuroprotective, no studies have examined whether this neuroprotection is directed at cerebral arterioles and no studies have examined whether activation of CB2 receptors can rescue cerebrovascular dysfunction during a chronic disease state such as type 1 diabetes (T1D). “Diabetes is a chronic disease associated with a high number of complications such as peripheral neuropathy, which causes sensorial disturbances and may lead to the development of diabetic neuropathic pain (DNP). The current treatment for DNP is just palliative and the drugs may cause severe adverse effects, leading to discontinuation of treatment. Thus, new therapeutic targets need to be urgently investigated.
“Diabetes is a chronic disease associated with a high number of complications such as peripheral neuropathy, which causes sensorial disturbances and may lead to the development of diabetic neuropathic pain (DNP). The current treatment for DNP is just palliative and the drugs may cause severe adverse effects, leading to discontinuation of treatment. Thus, new therapeutic targets need to be urgently investigated. “Chronic hepatitis C virus (HCV) infection is a risk factor of insulin resistance, and HCV-infected patients are at a high risk of developing diabetes.
“Chronic hepatitis C virus (HCV) infection is a risk factor of insulin resistance, and HCV-infected patients are at a high risk of developing diabetes. “Genus Cannabis belong to family Cannabaceae and is traditionally used as medicinal plant against many diseases notably asthma, malaria, treatment of skin diseases, diabetes and headache. The plant Cannabis sativa L. is flowering and an annual herbaceous plant located to eastern Asia but now of cosmopolitan distribution due to extensive cultivation.
“Genus Cannabis belong to family Cannabaceae and is traditionally used as medicinal plant against many diseases notably asthma, malaria, treatment of skin diseases, diabetes and headache. The plant Cannabis sativa L. is flowering and an annual herbaceous plant located to eastern Asia but now of cosmopolitan distribution due to extensive cultivation. “The prevalence rates of depression and anxiety are at least two times higher in diabetic patients, increasing morbidity and mortality.
“The prevalence rates of depression and anxiety are at least two times higher in diabetic patients, increasing morbidity and mortality.