
“Microglia, the dynamic innate immune cells of the central nervous system, become activated in epilepsy. The process of microglial activation in epilepsy results in the creation of an inflammatory environment around the site of seizure onset, which contributes to the epileptogenic process and epilepsy progression. Cannabidiol (CBD) has been effective for use as an adjunctive treatment for two severe pediatric seizure disorders. Newly recognized as an Food and Drug Administration (FDA)-approved drug treatment in epilepsy, it has gained in popularity primarily for pain management. Although CBD is readily available in stores and online retailers, its mechanism of action and specifically its effects on microglia and their functions are yet fully understood. In this study, we examine the effects of commercially available CBD on microglia inflammatory activation and neurogenic response, in the presence and absence of seizures. We use systemic administration of kainate to elicit seizures in mice, which are assessed behaviorally. Artisanal CBD is given in different modes of administration and timing to dissect its effect on seizure intensity, microglial activation and aberrant seizure-related neurogenesis. CBD significantly dampens microglial migration and accumulation to the hippocampus. While long term artisanal CBD use does not prevent or lessen seizure severity, CBD is a promising adjunctive partner for its ability to depress epileptogenic processes. These studies indicate that artisanal CBD is beneficial as it both decreases inflammation in the CNS and reduces the number of ectopic neurons deposited in the hippocampal area post seizure.”
https://pubmed.ncbi.nlm.nih.gov/35700815/
https://www.sciencedirect.com/science/article/abs/pii/S0306452222003049?via%3Dihub




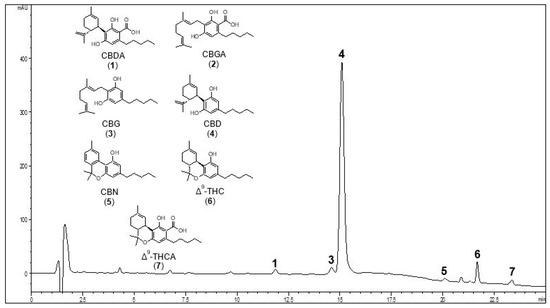
 “Cannabinoids have been found to be effective in controlling seizures and the highly purified form of cannabinoid derived for Cannabis sativa . Cannabidiol (CBD) is now approved for Lennox-Gastaut syndrome (LGS) and Dravet syndrome. CBD was used in a 9-year-old boy with LGS (unknown etiology) with very good results. The electroencephalography (EEG) response was very dramatic with near normalization of EEG background and complete control of seizures. The effect of CBD on EEG with such an improvement has not been described previously. Also, this adds to evidence that early intervention in LGS with CBD might be more helpful and improve outcomes.”
“Cannabinoids have been found to be effective in controlling seizures and the highly purified form of cannabinoid derived for Cannabis sativa . Cannabidiol (CBD) is now approved for Lennox-Gastaut syndrome (LGS) and Dravet syndrome. CBD was used in a 9-year-old boy with LGS (unknown etiology) with very good results. The electroencephalography (EEG) response was very dramatic with near normalization of EEG background and complete control of seizures. The effect of CBD on EEG with such an improvement has not been described previously. Also, this adds to evidence that early intervention in LGS with CBD might be more helpful and improve outcomes.” “Voltage-gated sodium channels are targets for a range of pharmaceutical drugs developed for treatment of neurological diseases.
“Voltage-gated sodium channels are targets for a range of pharmaceutical drugs developed for treatment of neurological diseases. “Pharmaceutically purified oral cannabidiol (CBD) has been recently approved by the US Food and Drug Administration and European Medicines Agency as treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS), which are severe and difficult-to-treat developmental and epileptic encephalopathies with onset in early childhood.
“Pharmaceutically purified oral cannabidiol (CBD) has been recently approved by the US Food and Drug Administration and European Medicines Agency as treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS), which are severe and difficult-to-treat developmental and epileptic encephalopathies with onset in early childhood. “In recent years there has been a growing appreciation by regulatory authorities that cannabis-based medicines can play a useful role in disease therapy.
“In recent years there has been a growing appreciation by regulatory authorities that cannabis-based medicines can play a useful role in disease therapy. “Highly purified cannabidiol (CBD) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut syndrome or Dravet syndrome in randomized, double-blind, add-on, controlled phase 3 trials.
“Highly purified cannabidiol (CBD) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut syndrome or Dravet syndrome in randomized, double-blind, add-on, controlled phase 3 trials.