
“Background: Purified cannabidiol (CBD), a non-psychoactive phytocannabinoid, has gained regulatory approval to treat intractable childhood epilepsies. Despite this, artisanal and commercial CBD-dominant hemp-based products continue to be used by epilepsy patients. Notably, the CBD doses used in these latter products are much lower than that found to be effective in reducing seizures in clinical trials with purified CBD. This might be because these CBD-dominant hemp products contain other bioactive compounds, including phytocannabinoids and terpenes, which may exert unique effects on epilepsy-relevant drug targets. Voltage-gated sodium (NaV) channels are vital for initiation of neuronal action potential propagation and genetic mutations in these channels result in epilepsy phenotypes. Recent studies suggest that NaV channels are inhibited by purified CBD. However, the effect of cannabis-based products on the function of NaV channels is unknown.
Methods: Using automated-planar patch-clamp technology, we profile a hemp-derived nutraceutical product (NP) against human NaV1.1-NaV1.8 expressed in mammalian cells to examine effects on the biophysical properties of channel conductance, steady-state fast inactivation and recovery from fast inactivation.
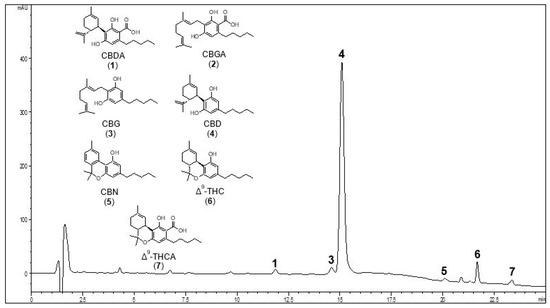
Results: NP modifies peak current amplitude of the NaV1.1-NaV1.7 subtypes and has variable effects on the biophysical properties for all channel subtypes tested. NP potently inhibits NaV channels revealing half-maximal inhibitory concentration (IC50) values of between 1.6 and 4.2 μg NP/mL. Purified CBD inhibits NaV1.1, NaV1.2, NaV1.6 and NaV1.7 to reveal IC50 values in the micromolar range. The CBD content of the product equates to IC50 values (93-245 nM), which are at least an order of magnitude lower than purified CBD. Unlike NP, hemp seed oil vehicle alone did not inhibit NaV channels, suggesting that the inhibitory effects of NP are independent of hemp seed oil.
Conclusions: This CBD-dominant NP potently inhibits NaV channels. Future study of the individual elements of NP, including phytocannabinoids and terpenes, may reveal a potent individual component or that its components interact to modulate NaV channels.”
https://pubmed.ncbi.nlm.nih.gov/35689251/
https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-022-00136-x



 “Cannabinoids have been found to be effective in controlling seizures and the highly purified form of cannabinoid derived for Cannabis sativa . Cannabidiol (CBD) is now approved for Lennox-Gastaut syndrome (LGS) and Dravet syndrome. CBD was used in a 9-year-old boy with LGS (unknown etiology) with very good results. The electroencephalography (EEG) response was very dramatic with near normalization of EEG background and complete control of seizures. The effect of CBD on EEG with such an improvement has not been described previously. Also, this adds to evidence that early intervention in LGS with CBD might be more helpful and improve outcomes.”
“Cannabinoids have been found to be effective in controlling seizures and the highly purified form of cannabinoid derived for Cannabis sativa . Cannabidiol (CBD) is now approved for Lennox-Gastaut syndrome (LGS) and Dravet syndrome. CBD was used in a 9-year-old boy with LGS (unknown etiology) with very good results. The electroencephalography (EEG) response was very dramatic with near normalization of EEG background and complete control of seizures. The effect of CBD on EEG with such an improvement has not been described previously. Also, this adds to evidence that early intervention in LGS with CBD might be more helpful and improve outcomes.” “Voltage-gated sodium channels are targets for a range of pharmaceutical drugs developed for treatment of neurological diseases.
“Voltage-gated sodium channels are targets for a range of pharmaceutical drugs developed for treatment of neurological diseases. “Pharmaceutically purified oral cannabidiol (CBD) has been recently approved by the US Food and Drug Administration and European Medicines Agency as treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS), which are severe and difficult-to-treat developmental and epileptic encephalopathies with onset in early childhood.
“Pharmaceutically purified oral cannabidiol (CBD) has been recently approved by the US Food and Drug Administration and European Medicines Agency as treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS), which are severe and difficult-to-treat developmental and epileptic encephalopathies with onset in early childhood. “In recent years there has been a growing appreciation by regulatory authorities that cannabis-based medicines can play a useful role in disease therapy.
“In recent years there has been a growing appreciation by regulatory authorities that cannabis-based medicines can play a useful role in disease therapy. “Highly purified cannabidiol (CBD) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut syndrome or Dravet syndrome in randomized, double-blind, add-on, controlled phase 3 trials.
“Highly purified cannabidiol (CBD) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut syndrome or Dravet syndrome in randomized, double-blind, add-on, controlled phase 3 trials. “Mutations in SYNGAP1 are associated with developmental delay, epilepsy, and autism spectrum disorder (ASD). Epilepsy is often drug-resistant in this syndrome with frequent drop attacks.
“Mutations in SYNGAP1 are associated with developmental delay, epilepsy, and autism spectrum disorder (ASD). Epilepsy is often drug-resistant in this syndrome with frequent drop attacks.