 “Background: Cannabis use is increasing, including among smokers, an at-risk population for cancer. Research is equivocal on whether using cannabis inhibits quitting cigarettes. The current longitudinal study investigated associations between smoking cannabis and subsequently quitting cigarettes.
“Background: Cannabis use is increasing, including among smokers, an at-risk population for cancer. Research is equivocal on whether using cannabis inhibits quitting cigarettes. The current longitudinal study investigated associations between smoking cannabis and subsequently quitting cigarettes.
Results: Adjusted cigarette quitting rates at follow-up did not differ significantly by baseline cannabis smoking status [never 36.2%, 95% confidence interval (CI), 34.5%-37.8%; former 34.1%, CI, 31.4%-37.0%; recent 33.6%, CI, 30.1%-37.3%], nor by frequency of cannabis smoking (low 31.4%, CI, 25.6%-37.3%; moderate 36.7%, CI, 30.7%-42.3%; high 34.4%, CI, 28.3%-40.2%) among recent baseline cannabis smokers. In cross-sectional analyses conducted at follow-up the proportion of cigarette smokers intending to quit smoking cigarettes in the next 30 days did not differ by cannabis smoking status (p=0.83).
Conclusions: Results do not support the hypothesis that cannabis smoking inhibits quitting cigarette smoking among adults.”
https://pubmed.ncbi.nlm.nih.gov/34348959/
“Results do not support the hypothesis that cannabis smoking inhibits quitting cigarette smoking among adults.” https://cebp.aacrjournals.org/content/early/2021/08/04/1055-9965.EPI-20-1810

 “Cannabis sativa
“Cannabis sativa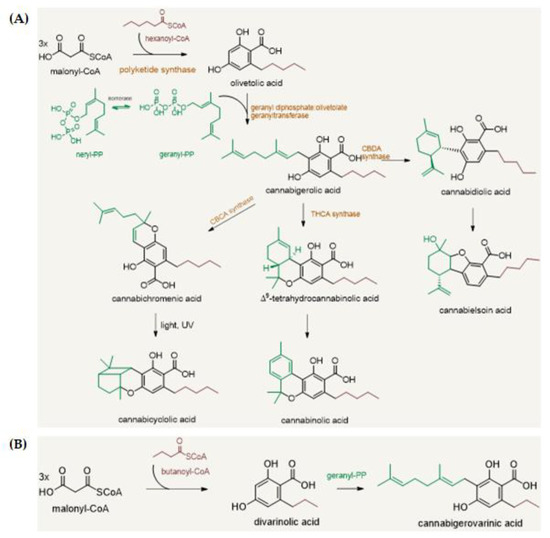
 “Considering the advantages of using medicinal herbs as supplementary treatments to sensitize conventional anti-cancer drugs, studying functional mechanisms and regulatory effects of Echinacea purpurea (as a non-cannabinoid plant)
“Considering the advantages of using medicinal herbs as supplementary treatments to sensitize conventional anti-cancer drugs, studying functional mechanisms and regulatory effects of Echinacea purpurea (as a non-cannabinoid plant) 


 “Providers need to be better equipped to discuss medical cannabis with patients even if they are not willing to prescribe it. The oncology community would be well served to ensure that providers are aware of existing cannabis research and are able to incorporate it into their communications with patients instead of leaving patients to figure out medical cannabis on their own.”
“Providers need to be better equipped to discuss medical cannabis with patients even if they are not willing to prescribe it. The oncology community would be well served to ensure that providers are aware of existing cannabis research and are able to incorporate it into their communications with patients instead of leaving patients to figure out medical cannabis on their own.” “In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”
“In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”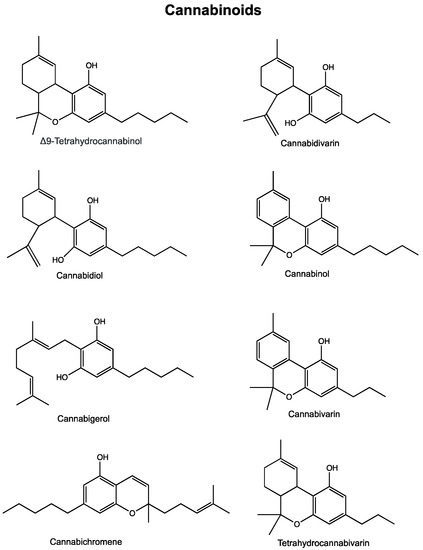
 “The recent announcement of marijuana legalization in Canada spiked many discussions about potential health benefits of Cannabis sativa.
“The recent announcement of marijuana legalization in Canada spiked many discussions about potential health benefits of Cannabis sativa.  “Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g.,
“Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g.,  “In recent years, the endocannabinoid system has received great interest as a potential therapeutic target in numerous pathological conditions.
“In recent years, the endocannabinoid system has received great interest as a potential therapeutic target in numerous pathological conditions. “Phytocannabinoids are unique terpenophenolic compounds predominantly produced in the glandular trichomes of the cannabis plant (Cannabis sativa L.). The delta-9- tetrahydrocannabinol (THC) is the main active constituent responsible for the plant’s psychoactive effect and, together with the non- psychoactive cannabidiol (CBD), the most investigated naturally occurring cannabinoid.
“Phytocannabinoids are unique terpenophenolic compounds predominantly produced in the glandular trichomes of the cannabis plant (Cannabis sativa L.). The delta-9- tetrahydrocannabinol (THC) is the main active constituent responsible for the plant’s psychoactive effect and, together with the non- psychoactive cannabidiol (CBD), the most investigated naturally occurring cannabinoid.