
“Historically, Cannabis is one of the first plants to be domesticated and used in medicine, though only in the last years the amount of Cannabis-based products or medicines has increased worldwide.
Previous preclinical studies and few published clinical trials have demonstrated the efficacy and safety of Cannabis-based medicines in humans. Indeed, Cannabis-related medicines are used to treat multiple pathological conditions, including neurodegenerative disorders.
In clinical practice, Cannabis products have already been introduced to treatment regimens of Alzheimer’s disease, Parkinson’s disease and Multiple Sclerosis’s patients, and the mechanisms of action behind the reported improvement in the clinical outcome and disease progression are associated with their anti-inflammatory, immunosuppressive, antioxidant, and neuroprotective properties, due to the modulation of the endocannabinoid system.
In this review, we describe the role played by the endocannabinoid system in the physiopathology of Alzheimer, Parkinson, and Multiple Sclerosis, mainly at the neuroimmunological level. We also discuss the evidence for the correlation between phytocannabinoids and their therapeutic effects in these disorders, thus describing the main clinical studies carried out so far on the therapeutic performance of Cannabis-based medicines.”
https://pubmed.ncbi.nlm.nih.gov/35707521/
“Based on scientific evidence, the use of Cannabis-based products or Cannabis-based medicine (CBM) has been growing among patients diagnosed with neurodegenerative diseases. Most reports of clinical trials also describe significant improvement in disease-related primary and/or secondary symptoms, besides general improvement in life quality.”
https://www.frontiersin.org/articles/10.3389/fncel.2022.917164/full


 “Cannabis sativa
“Cannabis sativa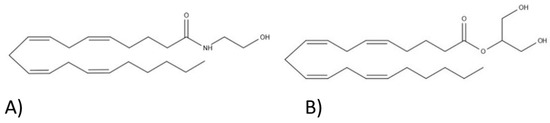
 “Background and purpose:
“Background and purpose:  “In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite.
“In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite. 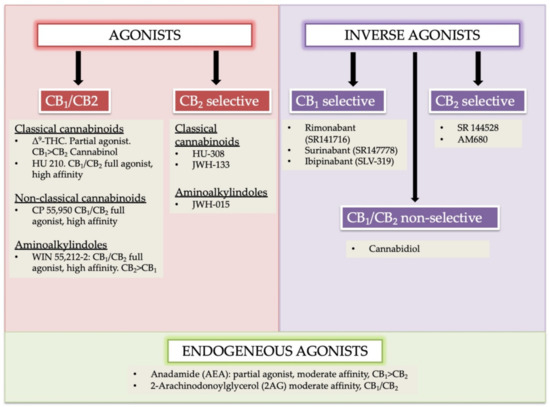
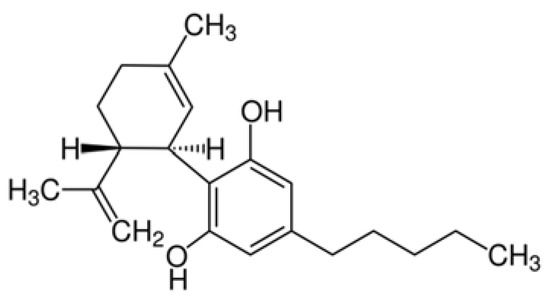
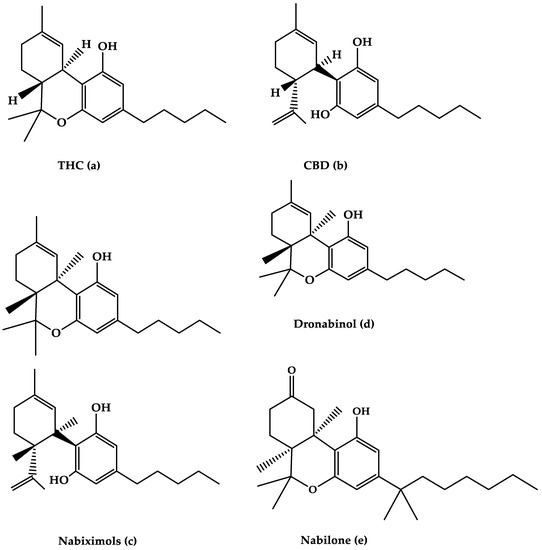
 “Medical cannabis and individual cannabinoids, such as tetrahydrocannabinol (THC) and cannabidiol (CBD), are receiving growing attention in both the media and the scientific literature. The Cannabis plant, however, produces over 100 different cannabinoids, and cannabigerol (CBG) serves as the precursor molecule for the most abundant phytocannabinoids.
“Medical cannabis and individual cannabinoids, such as tetrahydrocannabinol (THC) and cannabidiol (CBD), are receiving growing attention in both the media and the scientific literature. The Cannabis plant, however, produces over 100 different cannabinoids, and cannabigerol (CBG) serves as the precursor molecule for the most abundant phytocannabinoids. “The aberrant accumulation of disease-specific protein aggregates accompanying cognitive decline is a pathological hallmark of age-associated neurological disorders, also termed as proteinopathies, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis.
“The aberrant accumulation of disease-specific protein aggregates accompanying cognitive decline is a pathological hallmark of age-associated neurological disorders, also termed as proteinopathies, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis.