
“Background: The legalization of cannabis in several states has led to increased documented use in the population. Bariatric surgery patients are no exception with estimates of anywhere from 6 to 8%. Cannabis is known to be associated with increased appetite, mood disorders, hyperphagia, and rarely, hyperemesis, which can potentially affect post-surgical weight loss. We aim to study the differences in bariatric surgery outcomes between cannabis users and non-users.
Results: A cohort of 364 sleeve gastrectomy patients met inclusion criteria, 31 (8.5%) CU and 333 (91.5%) non-CU. There was no difference in EWL between CU and non-CU at 1 week, 1 month, 3 months, 6 months, 9 months, 1 year, and 2 years. However, the CU group trended towards greater EWL at 3 years (52.9% vs. 38.1%, p = 0.094) and at 5 years (49.8% vs. 32.7%, p = 0.068). There were no significant differences between CU and non-CU with respect to either incidence or severity of PONV at one year after surgery or longer follow-up.
Conclusion: Cannabis users did not experience inferior weight loss after bariatric surgery despite common assumptions that appetite stimulation can lead to suboptimal weight loss outcomes. Our findings add to other work challenging this dogma. Larger, long-term, multicenter studies are warranted.”
https://pubmed.ncbi.nlm.nih.gov/35861881/
https://link.springer.com/article/10.1007/s00464-022-09453-x



 “Chronic hepatitis B virus (HBV) infection may evolve into cirrhosis and hepatocellular carcinoma, and this progression may be accelerated by specific risk factors, including overweight and obesity. Although evidence for a protective effect of cannabis use on elevated body weight has been found for other populations, no data are available for HBV-infected patients.
“Chronic hepatitis B virus (HBV) infection may evolve into cirrhosis and hepatocellular carcinoma, and this progression may be accelerated by specific risk factors, including overweight and obesity. Although evidence for a protective effect of cannabis use on elevated body weight has been found for other populations, no data are available for HBV-infected patients.  “Interest in CBG (cannabigerol) has been growing in the past few years, due to its anti-inflammatory properties and other therapeutic benefits.
“Interest in CBG (cannabigerol) has been growing in the past few years, due to its anti-inflammatory properties and other therapeutic benefits. 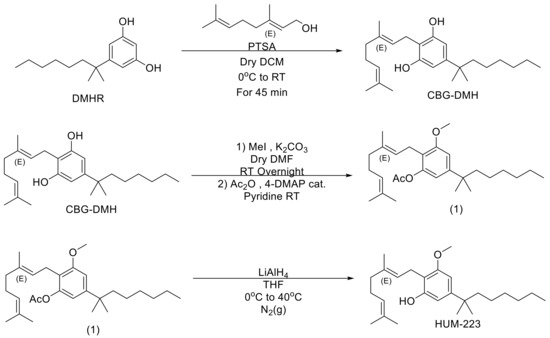
 “Obesity-related insulin resistance (IR) and attenuated brain insulin signaling are significant risk factors for neurodegenerative disorders, e.g., Alzheimer’s disease. IR and type 2 diabetes correlate with an increased concentration of sphingolipids, a class of lipids that play an essential structural role in cellular membranes and cell signaling pathways.
“Obesity-related insulin resistance (IR) and attenuated brain insulin signaling are significant risk factors for neurodegenerative disorders, e.g., Alzheimer’s disease. IR and type 2 diabetes correlate with an increased concentration of sphingolipids, a class of lipids that play an essential structural role in cellular membranes and cell signaling pathways.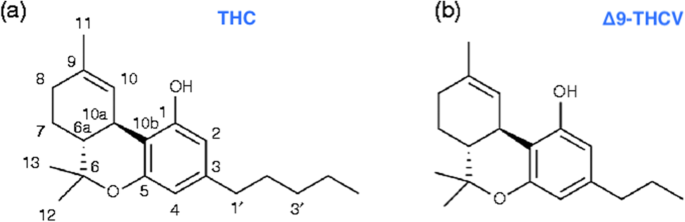 “Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects.
“Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects.  “(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants.
“(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants.