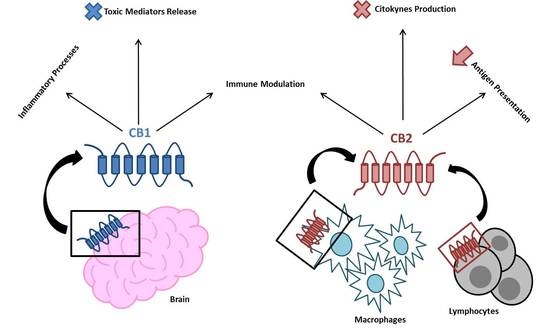
 “Endocannabinoid system consists of cannabinoid type 1 (CB1) and cannabinoid type 2 (CB2) receptors, their endogenous ligands, and the enzymes responsible for their synthesis and degradation. CB2, to a great extent, and CB1, to a lesser extent, are involved in regulating the immune response. They also regulate the inflammatory processes by inhibiting pro-inflammatory mediator release and immune cell proliferation. This review provides an overview on the role of the endocannabinoid system with a major focus on cannabinoid receptors in the pathogenesis and onset of inflammatory and autoimmune pediatric diseases, such as immune thrombocytopenia, juvenile idiopathic arthritis, inflammatory bowel disease, celiac disease, obesity, neuroinflammatory diseases, and type 1 diabetes mellitus. These disorders have a high social impact and represent a burden for the healthcare system, hence the importance of individuating more innovative and effective treatments. The endocannabinoid system could address this need, representing a possible new diagnostic marker and therapeutic target.”
“Endocannabinoid system consists of cannabinoid type 1 (CB1) and cannabinoid type 2 (CB2) receptors, their endogenous ligands, and the enzymes responsible for their synthesis and degradation. CB2, to a great extent, and CB1, to a lesser extent, are involved in regulating the immune response. They also regulate the inflammatory processes by inhibiting pro-inflammatory mediator release and immune cell proliferation. This review provides an overview on the role of the endocannabinoid system with a major focus on cannabinoid receptors in the pathogenesis and onset of inflammatory and autoimmune pediatric diseases, such as immune thrombocytopenia, juvenile idiopathic arthritis, inflammatory bowel disease, celiac disease, obesity, neuroinflammatory diseases, and type 1 diabetes mellitus. These disorders have a high social impact and represent a burden for the healthcare system, hence the importance of individuating more innovative and effective treatments. The endocannabinoid system could address this need, representing a possible new diagnostic marker and therapeutic target.”
https://www.ncbi.nlm.nih.gov/pubmed/31771129
https://www.mdpi.com/1422-0067/20/23/5875

 “Medicinal
“Medicinal 


 “Obesity, an important risk factor for developing chronic kidney disease (CKD), affects the kidneys by two main molecular signaling pathways: the endocannabinoid/CB1 R system, whose activation in obesity promotes renal inflammation, fibrosis, and injury; and the inducible nitric oxide synthase (iNOS), which generates reactive oxygen species resulting in oxidative stress. Hence, a combined peripheral inhibitory molecule that targets both CB1 R and iNOS may serve as an efficacious therapeutic agent against obesity-induced CKD.
“Obesity, an important risk factor for developing chronic kidney disease (CKD), affects the kidneys by two main molecular signaling pathways: the endocannabinoid/CB1 R system, whose activation in obesity promotes renal inflammation, fibrosis, and injury; and the inducible nitric oxide synthase (iNOS), which generates reactive oxygen species resulting in oxidative stress. Hence, a combined peripheral inhibitory molecule that targets both CB1 R and iNOS may serve as an efficacious therapeutic agent against obesity-induced CKD. “Small molecules targeting peripheral CB1 receptors have therapeutic potential in a variety of disorders including obesity-related, hormonal and metabolic abnormalities, while avoiding the psychoactive effects in the CNS.
“Small molecules targeting peripheral CB1 receptors have therapeutic potential in a variety of disorders including obesity-related, hormonal and metabolic abnormalities, while avoiding the psychoactive effects in the CNS. “Obese individuals are more likely to show insulin resistance (IR). However, limited population studies on
“Obese individuals are more likely to show insulin resistance (IR). However, limited population studies on 
 “The
“The  “Healthy aging includes freedom from disease, ability to engage in physical activity, and maintenance of cognitive skills for which diet is a major lifestyle factor. Aging, diet, and health are at the forefront of well-being for the growing population of older adults with the caveat of reducing and controlling pain. Obesity and diabetes risk increase in frequency in adults, and exercise is encouraged to control weight, reduce risk of type II diabetes, and maintain muscle mass and mobility.
“Healthy aging includes freedom from disease, ability to engage in physical activity, and maintenance of cognitive skills for which diet is a major lifestyle factor. Aging, diet, and health are at the forefront of well-being for the growing population of older adults with the caveat of reducing and controlling pain. Obesity and diabetes risk increase in frequency in adults, and exercise is encouraged to control weight, reduce risk of type II diabetes, and maintain muscle mass and mobility.