“Preclinical models provided ample evidence that cannabinoids are cytotoxic against cancer cells. Among the best studied phytocannabinoids, cannabidiol (CBD) is most promising for the treatment of cancer as it lacks the psychotomimetic properties of delta-9-tetrahydrocannabinol (THC). In vitro studies and animal experiments point to a concentration- (dose-)dependent anticancer effect. The effectiveness of pure compounds versus extracts is the subject of an ongoing debate. Actual results demonstrate that CBD-rich hemp extracts must be distinguished from THC-rich cannabis preparations. Whereas pure CBD was superior to CBD-rich extracts in most in vitro experiments, the opposite was observed for pure THC and THC-rich extracts, although exceptions were noted. The cytotoxic effects of CBD, THC and extracts seem to depend not only on the nature of cannabinoids and the presence of other phytochemicals but also largely on the nature of cell lines and test conditions. Neither CBD nor THC are universally efficacious in reducing cancer cell viability. The combination of pure cannabinoids may have advantages over single agents, although the optimal ratio seems to depend on the nature of cancer cells; the existence of a ‘one size fits all’ ratio is very unlikely. As cannabinoids interfere with the endocannabinoid system (ECS), a better understanding of the circadian rhythmicity of the ECS, particularly endocannabinoids and receptors, as well as of the rhythmicity of biological processes related to the growth of cancer cells, could enhance the efficacy of a therapy with cannabinoids by optimization of the timing of the administration, as has already been reported for some of the canonical chemotherapeutics. Theoretically, a CBD dose administered at noon could increase the peak of anandamide and therefore the effects triggered by this agent. Despite the abundance of preclinical articles published over the last 2 decades, well-designed controlled clinical trials on CBD in cancer are still missing. The number of observations in cancer patients, paired with the anticancer activity repeatedly reported in preclinical in vitro and in vivo studies warrants serious scientific exploration moving forward.”
Category Archives: Ovarian Cancer
Cannabis as a potential compound against various malignancies, legal aspects, advancement by exploiting nanotechnology and clinical trials
“Various preclinical and clinical studies exhibited the potential of cannabis against various diseases, including cancer and related pain. Subsequently, many efforts have been made to establish and develop cannabis-related products and make them available as prescription products. Moreover, FDA has already approved some cannabis-related products, and more advancement in this aspect is still going on. However, the approved product of cannabis is in oral dosage form, which exerts various limitations to achieve maximum therapeutic effects. A considerable translation is on a hike to improve bioavailability, and ultimately, the therapeutic efficacy of cannabis by the employment of nanotechnology. Besides the well-known psychotropic effects of cannabis upon the use at high doses, literature has also shown the importance of cannabis and its constituents in minimising the lethality of cancer in the preclinical models. This review discusses the history of cannabis, its legal aspect, safety profile, the mechanism by which cannabis combats with cancer, and the advancement of clinical therapy by exploiting nanotechnology. A brief discussion related to the role of cannabinoid in various cancers has also been incorporated. Lastly, the information regarding completed and ongoing trials have also been elaborated.”
KY Hemp-induced Modulation of Ovarian Cancer Cell Metastasis
 “Our laboratory is interested in searching for a new plant-based therapeutics to treat ovarian cancer.
“Our laboratory is interested in searching for a new plant-based therapeutics to treat ovarian cancer.
We are interested in studying anti-cancer effects of KY grown hemp as a potential candidate drug.
Marijuana and hemp belong to the same genus and species. However, they are different in cannabidiol (CBD) and tetrahydrocannabinol (THC) content.
Both CBD and THC are therapeutically beneficial. Hemp is harmless and non-addictive.
Major objective of this study is to investigate whether KY hemp extract can modulate the metastasis of ovarian cancer.
Based on the data here we conclude that KY hemp has significant anti-metastatic properties against ovarian cancer.”
https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.2018.32.1_supplement.667.7
Searching for a New Anti-Cancer Drug: Investigation of KY Hemp-Induced Apoptosis in Ovarian Cancer Cells

“Marijuana (cannabis sativa) is a schedule 1 drug that has been recently approved by some states in the US for its therapeutic benefit.
Although there are a few reports about its anti-cancer potential, currently it has been used mainly for treatment-resistant epilepsy and to alleviate pain.
Hemp, which belongs to the same genus and species as marijuana, shows similar therapeutic benefits without addictive potential.
Our laboratory is interested in examining for unconventional therapies for ovarian cancer.
The main objective of the current study is to investigate hemp-induced modulation of A2780 ovarian cancer cell apoptosis.
Based on the data here we conclude that KY hemp has anti-cancer potential against ovarian cancer.”
https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.2018.32.1_supplement.616.1
Education and communication are critical to effectively incorporating cannabis into cancer treatment
 “Providers need to be better equipped to discuss medical cannabis with patients even if they are not willing to prescribe it. The oncology community would be well served to ensure that providers are aware of existing cannabis research and are able to incorporate it into their communications with patients instead of leaving patients to figure out medical cannabis on their own.”
“Providers need to be better equipped to discuss medical cannabis with patients even if they are not willing to prescribe it. The oncology community would be well served to ensure that providers are aware of existing cannabis research and are able to incorporate it into their communications with patients instead of leaving patients to figure out medical cannabis on their own.”
https://pubmed.ncbi.nlm.nih.gov/32986251/
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.33204
Cancer patients’ experiences with medicinal cannabis-related care
 “Background: Little is known about medical cannabis (MC)-related care for patients with cancer using MC.
“Background: Little is known about medical cannabis (MC)-related care for patients with cancer using MC.
Methods: Semistructured telephone interviews were conducted in a convenience sample of individuals (n = 24) with physician-confirmed oncologic diagnoses and state/district authorization to use MC (Arizona, California, Florida, Illinois, Massachusetts, Oregon, New York, and Washington, DC) from April 2017 to March 2019. Standard qualitative techniques were used to assess the degree of MC-related health care oversight, MC practices, and key information sources.
Results: Among 24 participants (median age, 57 years; range, 30-71 years; 16 women [67%]), MC certifications were typically issued by a professional new to a patient’s care after a brief, perfunctory consultation. Patients disclosed MCuse to their established medical teams but received little medical advice about whether and how to use MC. Patients with cancer used MC products as multipurpose symptom management and as cancer-directed therapy, sometimes in lieu of standard-of-care treatments. Personal experimentation, including methodical self-monitoring, was an important source of MC know-how. Absent formal advice from medical professionals, patients relied on nonmedical sources for MC information.
Conclusions: Patients with cancer used MC with minimal medical oversight. Most received MC certifications through brief meetings with unfamiliar professionals. Participants desired but were often unable to access high-quality clinical information about MC from their established medical teams. Because many patients are committed to using MC, a product sustained by a growing industry, medical providers should familiarize themselves with the existing data for MM and its limitations to address a poorly met clinical need.”
https://pubmed.ncbi.nlm.nih.gov/32986266/
“Notably, oncology patients reported using medical cannabis (MC) for symptom management and as cancer‐directed therapy, sometimes instead of traditional treatments.”
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.33202
Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis
 “In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”
“In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”
https://pubmed.ncbi.nlm.nih.gov/32708138/
https://www.mdpi.com/2072-6694/12/7/1985
Enhancing ovarian cancer conventional chemotherapy through the combination with cannabidiol loaded microparticles
 “In this work, we evaluated, for the first time, the antitumor effect of cannabidiol (CBD) as monotherapy and in combination with conventional chemotherapeutics in ovarian cancer and developed PLGA-microparticles as CBD carriers to optimize its anticancer activity.
“In this work, we evaluated, for the first time, the antitumor effect of cannabidiol (CBD) as monotherapy and in combination with conventional chemotherapeutics in ovarian cancer and developed PLGA-microparticles as CBD carriers to optimize its anticancer activity.
Spherical microparticles, with a mean particle size around 25 µm and high entrapment efficiency were obtained. Microparticles elaborated with a CBD:polymer ratio of 10:100 were selected due to the most suitable release profile with a zero-order CBD release (14.13±0.17 μg/day/10 mg Mps) for 40 days.
The single administration of this formulation showed an in vitro extended antitumor activity for at least 10 days and an in ovo antitumor efficacy comparable to that of CBD in solution after daily topical administration (≈1.5-fold reduction in tumor growth vs control). The use of CBD in combination with paclitaxel (PTX) was really effective.
The best treatment schedule was the pre+co-administration of CBD (10µM) with PTX. Using this protocol, the single administration of microparticles was even more effective than the daily administration of CBD in solution, achieving a ≈10- and 8- fold reduction in PTX IC50 respectively. This protocol was also effective in ovo. While PTX conducted to a 1.5-fold tumor growth inhibition, its combination with both CBD in solution (daily administered) and 10-Mps (single administration) showed a 2-fold decrease.
These results show the promising potential of CBD-Mps administered in combination with PTX for ovarian cancer treatment, since it would allow to reduce the administered dose of this antineoplastic drug maintaining the same efficacy and, as a consequence, reducing PTX adverse effects.”
https://pubmed.ncbi.nlm.nih.gov/32682943/
https://www.sciencedirect.com/science/article/abs/pii/S0939641120302113?via%3Dihub

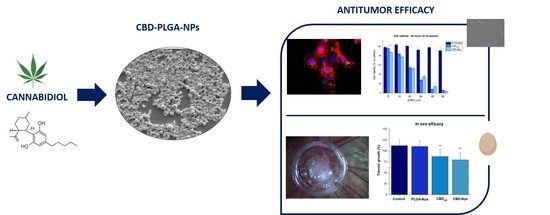
PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and In Ovo Assessment.
 “The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
“The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
Cannabidiol (CBD), the main nonpsychotropic cannabinoid, appears as a potential anticancer drug. The aim of this work was to develop polymer nanoparticles as CBD carriers capable of being internalised by ovarian cancer cells.
The drug-loaded nanoparticles (CBD-NPs) exhibited a spherical shape, a particle size around 240 nm and a negative zeta potential (-16.6 ± 1.2 mV). The encapsulation efficiency was high, with values above 95%. A controlled CBD release for 96 h was achieved. Nanoparticle internalisation in SKOV-3 epithelial ovarian cancer cells mainly occurred between 2 and 4 h of incubation. CBD antiproliferative activity in ovarian cancer cells was preserved after encapsulation. In fact, CBD-NPs showed a lower IC50 values than CBD in solution. Both CBD in solution and CBD-NPs induced the expression of PARP, indicating the onset of apoptosis. In SKOV-3-derived tumours formed in the chick embryo model, a slightly higher-although not statistically significant-tumour growth inhibition was observed with CBD-NPs compared to CBD in solution.
To sum up, poly-lactic-co-glycolic acid (PLGA) nanoparticles could be a good strategy to deliver CBD intraperitoneally for ovarian cancer treatment.”
https://www.ncbi.nlm.nih.gov/pubmed/32397428
https://www.mdpi.com/1999-4923/12/5/439
Cannabinoids as anticancer therapeutic agents.
 “The recent announcement of marijuana legalization in Canada spiked many discussions about potential health benefits of Cannabis sativa. Cannabinoids are active chemical compounds produced by cannabis, and their numerous effects on the human body are primarily exerted through interactions with cannabinoid receptor types 1 (CB1) and 2 (CB2). Cannabinoids are broadly classified as endo-, phyto-, and synthetic cannabinoids. In this review, we will describe the activity of cannabinoids on the cellular level, comprehensively summarize the activity of all groups of cannabinoids on various cancers and propose several potential mechanisms of action of cannabinoids on cancer cells.”
“The recent announcement of marijuana legalization in Canada spiked many discussions about potential health benefits of Cannabis sativa. Cannabinoids are active chemical compounds produced by cannabis, and their numerous effects on the human body are primarily exerted through interactions with cannabinoid receptor types 1 (CB1) and 2 (CB2). Cannabinoids are broadly classified as endo-, phyto-, and synthetic cannabinoids. In this review, we will describe the activity of cannabinoids on the cellular level, comprehensively summarize the activity of all groups of cannabinoids on various cancers and propose several potential mechanisms of action of cannabinoids on cancer cells.”
https://www.ncbi.nlm.nih.gov/pubmed/32249682
“Endocannabinoids and phytocannabinoids can be used for cancer therapy. Cannabis extracts have stronger anti-tumor capacity than single cannabinoids. Combination of several cannabinoids may have more potent effect on cancer.”
https://www.tandfonline.com/doi/abs/10.1080/15384101.2020.1742952?journalCode=kccy20
