 “Cannabidiol (CBD) is a major chemical compound present in Cannabis sativa.
“Cannabidiol (CBD) is a major chemical compound present in Cannabis sativa.
CBD is a nonpsychotomimetic substance, and it is considered one of the most promising candidates for the treatment of psychiatric disorders.
The aim of this review is to illustrate the state of art about scientific research and the evidence of effectiveness of CBD in psychiatric patients.
RESULTS:
Preclinical and clinical studies on potential role of CBD in psychiatry were collected and further discussed. We found four clinical studies describing the effects of CBD in psychiatric patients: two studies about schizophrenic patients and the other two studies carried out on CBD effects in patients affected by generalized social anxiety disorder (SAD).
CONCLUSION:
Results from these studies are promising and suggest that CBD may have a role in the development of new therapeutic strategies in mental diseases, and they justify an in-depth commitment in this field. However, clinical evidence we show for CBD in psychiatric patients is instead still poor and limited to schizophrenia and anxiety, and it needs to be implemented with further studies carried out on psychiatric patients.”
https://www.ncbi.nlm.nih.gov/pubmed/31558911
“Results of our research, enriched in assessment of methodological quality of the studies, confirm the view of this cannabinoid as a promising molecule especially in particular sectors of psychiatry such as schizophrenia, anxiety, depression, and autism. CBD is considered a safe substance and is one of the most promising candidates for the treatment of psychiatric disorders”.

 “Accumulating evidence points towards the antipsychotic potential of
“Accumulating evidence points towards the antipsychotic potential of 
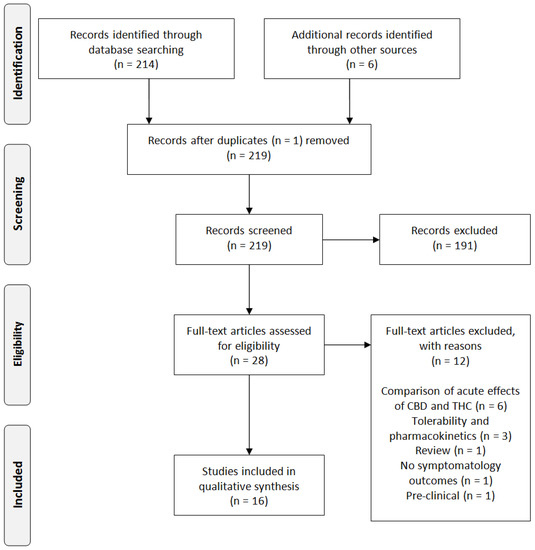
 “Cognitive impairment is a major source of disability in schizophrenia and current antipsychotic drugs (APDs) have minimal efficacy for this symptom domain.
“Cognitive impairment is a major source of disability in schizophrenia and current antipsychotic drugs (APDs) have minimal efficacy for this symptom domain. “The present findings reveal an imbalance in the expression and function of different elements of the endocannabinoid system in schizophrenia.
“The present findings reveal an imbalance in the expression and function of different elements of the endocannabinoid system in schizophrenia.

