 “Diabetes is a chronic disease associated with a high number of complications such as peripheral neuropathy, which causes sensorial disturbances and may lead to the development of diabetic neuropathic pain (DNP). The current treatment for DNP is just palliative and the drugs may cause severe adverse effects, leading to discontinuation of treatment. Thus, new therapeutic targets need to be urgently investigated.
“Diabetes is a chronic disease associated with a high number of complications such as peripheral neuropathy, which causes sensorial disturbances and may lead to the development of diabetic neuropathic pain (DNP). The current treatment for DNP is just palliative and the drugs may cause severe adverse effects, leading to discontinuation of treatment. Thus, new therapeutic targets need to be urgently investigated.
Studies have shown that cannabinoids have promising effects in the treatment of several pathological conditions, including chronic pain.
Thus, we aimed to investigate the acute effect of the intrathecal injection of CB1 or CB2 cannabinoid receptor agonists N-(2-chloroethyl)-5Z, 8Z, 11Z, 14Z-eicosatetraenamide (ACEA) or JWH 133, respectively (10, 30 or 100 μg/rat) on the mechanical allodynia associated with experimental diabetes induced by streptozotocin (60 mg/kg; intraperitoneal) in rats.
Cannabinoid receptor antagonists CB1 AM251 or CB2 AM630 (1 mg/kg) were given before treatment with respective agonists to confirm the involvement of cannabinoid CB1 or CB2 receptors. Rats with diabetes exhibited a significant reduction on the paw mechanical threshold 2 weeks after diabetes induction, having the maximum effect observed 4 weeks after the streptozotocin injection. This mechanical allodynia was significantly improved by intrathecal treatment with ACEA or JWH 133 (only at the higher dose of 100 μg). Pre-treatment with AM251 or AM630 significantly reverted the anti-allodynic effect of the ACEA or JWH 133, respectively.
Considering the clinical challenge that the treatment of DPN represents, this study showed for the first time, that the intrathecal cannabinoid receptors agonists may represent an alternative for the treatment of DNP.”

 “The contribution of substance use disorders to the burden of severe maternal morbidity in the United States is poorly understood. The objective was to estimate the independent association between substance use disorders during pregnancy and risk of severe maternal morbidity.
“The contribution of substance use disorders to the burden of severe maternal morbidity in the United States is poorly understood. The objective was to estimate the independent association between substance use disorders during pregnancy and risk of severe maternal morbidity. “Although several lines of evidence support the hypothesis of a dysregulation of serotoninergic neurotransmission in the pathophysiology of obsessive-compulsive disorder (OCD), there is also evidence for an involvement of other pathways such as the GABAergic, glutamatergic, and dopaminergic systems.
“Although several lines of evidence support the hypothesis of a dysregulation of serotoninergic neurotransmission in the pathophysiology of obsessive-compulsive disorder (OCD), there is also evidence for an involvement of other pathways such as the GABAergic, glutamatergic, and dopaminergic systems. “Posttraumatic stress disorder (PTSD) may stem from the formation of aberrant and enduring aversive memories. Some PTSD patients have recreationally used Cannabis, probably aiming at relieving their symptomatology.
“Posttraumatic stress disorder (PTSD) may stem from the formation of aberrant and enduring aversive memories. Some PTSD patients have recreationally used Cannabis, probably aiming at relieving their symptomatology. “Neurological disorders such as neurodegenerative diseases or traumatic brain injury are associated with cognitive, motor and behavioural changes that influence the quality of life of the patients. Although different therapeutic strategies have been developed and tried until now to decrease the neurological decline, no treatment has been found to cure these pathologies.
“Neurological disorders such as neurodegenerative diseases or traumatic brain injury are associated with cognitive, motor and behavioural changes that influence the quality of life of the patients. Although different therapeutic strategies have been developed and tried until now to decrease the neurological decline, no treatment has been found to cure these pathologies.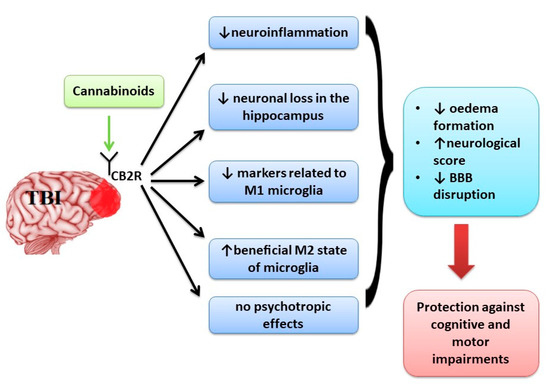
 “Few studies to date have measured the real-time effects of consumption of common and commercially available Cannabis products for the treatment of headache and migraine under naturalistic conditions. This study examines, for the first time, the effectiveness of using dried Cannabis flower, the most widely used type of Cannabis product in the United States, in actual time for treatment of headache- and migraine-related pain and the associations between different product characteristics and changes in symptom intensity following Cannabis use.
“Few studies to date have measured the real-time effects of consumption of common and commercially available Cannabis products for the treatment of headache and migraine under naturalistic conditions. This study examines, for the first time, the effectiveness of using dried Cannabis flower, the most widely used type of Cannabis product in the United States, in actual time for treatment of headache- and migraine-related pain and the associations between different product characteristics and changes in symptom intensity following Cannabis use. “Cannabinoid CB2 receptor (CB2) agonists are potential analgesics void of psychotropic effects.
“Cannabinoid CB2 receptor (CB2) agonists are potential analgesics void of psychotropic effects. “Chronic ethanol abuse can lead to harmful consequences for the heart, resulting in systolic dysfunction, variability in the heart rate, arrhythmia, and cardiac remodelling. However, the precise molecular mechanism responsible for ethanol-induced cardiomyopathy is poorly understood. In this regard, the present study aimed to describe the RIP1/RIP3/MLKL-mediated necroptotic cell death that may be involved in ethanol-induced cardiomyopathy and characterize CBR-mediated effects on the signalling pathway and myocardial injury.
“Chronic ethanol abuse can lead to harmful consequences for the heart, resulting in systolic dysfunction, variability in the heart rate, arrhythmia, and cardiac remodelling. However, the precise molecular mechanism responsible for ethanol-induced cardiomyopathy is poorly understood. In this regard, the present study aimed to describe the RIP1/RIP3/MLKL-mediated necroptotic cell death that may be involved in ethanol-induced cardiomyopathy and characterize CBR-mediated effects on the signalling pathway and myocardial injury. “Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
“Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.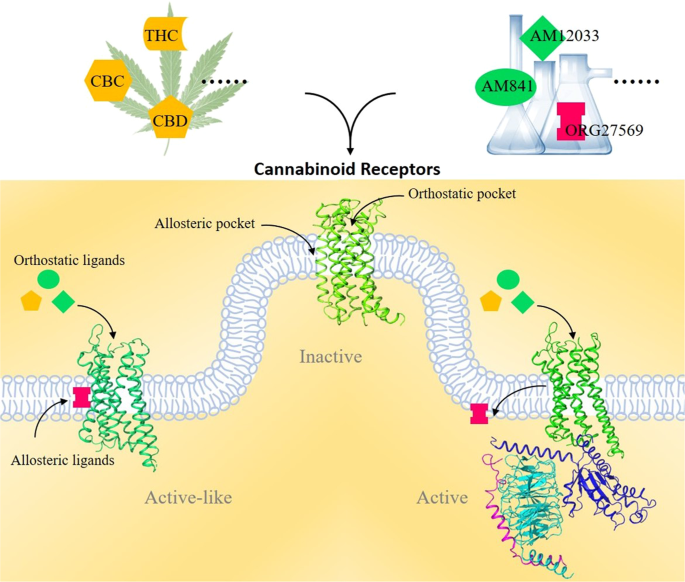
 “Thirty years ago, the discovery of a cannabinoid (CB) receptor that interacts with the psychoactive compound in Cannabis led to the identification of anandamide, an endogenous receptor ligand or endocannabinoid. Research on endocannabinoids has since exploded, and additional receptors along with their lipid mediators and signaling pathways continue to be revealed. Specifically, in humans, the release of endocannabinoids from membrane lipids occurs on demand and the signaling process is rapidly attenuated by the breakdown of the ligand suggesting a tight regulation of the endocannabinoid system (ECS). Additionally, the varying distribution of CB receptors between the central nervous system and other tissues allows for the ECS to participate in a wide range of cognitive and physiological processes. Select plant-derived ‘phyto’cannabinoids such as Δ-9-tetrahydrocannabinol (Δ9-THC) bind to the CB receptors and trigger the ECS, and in the case of Δ9-THC, while it has therapeutic value, can also produce detrimental effects. Current research is aimed at the identification of additional phytocannabinoids with minimal psychotropic effects with potential for therapeutic development. Although decades of research on the ECS and its components have expanded our understanding of the mechanisms and implications of endocannabinoid signaling in mammals, it continues to evolve. Here, we provide a brief overview of the ECS and its overlap with other related lipid-mediated signaling pathways.”
“Thirty years ago, the discovery of a cannabinoid (CB) receptor that interacts with the psychoactive compound in Cannabis led to the identification of anandamide, an endogenous receptor ligand or endocannabinoid. Research on endocannabinoids has since exploded, and additional receptors along with their lipid mediators and signaling pathways continue to be revealed. Specifically, in humans, the release of endocannabinoids from membrane lipids occurs on demand and the signaling process is rapidly attenuated by the breakdown of the ligand suggesting a tight regulation of the endocannabinoid system (ECS). Additionally, the varying distribution of CB receptors between the central nervous system and other tissues allows for the ECS to participate in a wide range of cognitive and physiological processes. Select plant-derived ‘phyto’cannabinoids such as Δ-9-tetrahydrocannabinol (Δ9-THC) bind to the CB receptors and trigger the ECS, and in the case of Δ9-THC, while it has therapeutic value, can also produce detrimental effects. Current research is aimed at the identification of additional phytocannabinoids with minimal psychotropic effects with potential for therapeutic development. Although decades of research on the ECS and its components have expanded our understanding of the mechanisms and implications of endocannabinoid signaling in mammals, it continues to evolve. Here, we provide a brief overview of the ECS and its overlap with other related lipid-mediated signaling pathways.”