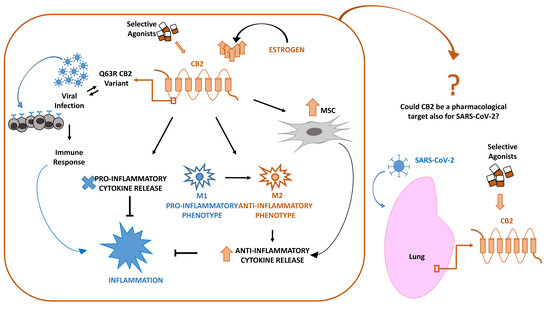
“The worldwide prevalence of neurological and neurodegenerative disorders, such as depression or Alzheimer’s disease, has spread extensively throughout the last decades, becoming an enormous health issue.
“The worldwide prevalence of neurological and neurodegenerative disorders, such as depression or Alzheimer’s disease, has spread extensively throughout the last decades, becoming an enormous health issue.
Numerous data indicate a distinct correlation between the altered endocannabinoid signaling and different aspects of brain physiology, such as memory or neurogenesis. Moreover, the endocannabinoid system is widely regarded as a crucial factor in the development of neuropathologies. Thus, targeting those disorders via synthetic cannabinoids, as well as phytocannabinoids, becomes a widespread research issue.
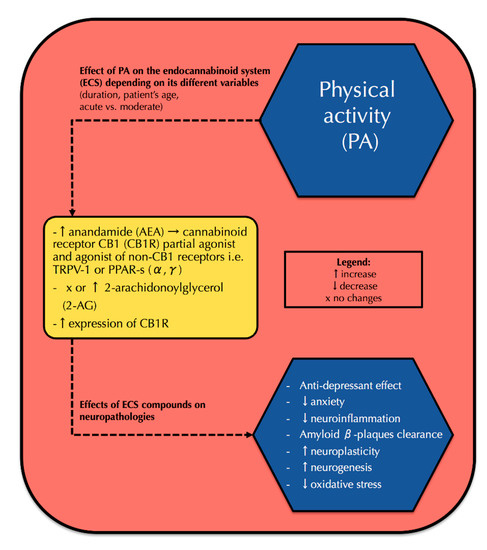
Over the last decade, the endocannabinoid system has been extensively studied for its correlation with physical activity. Recent data showed that physical activity correlates with elevated endocannabinoid serum concentrations and increased cannabinoid receptor type 1 (CB1R) expression in the brain, which results in positive neurological effects including antidepressant effect, ameliorated memory, neuroplasticity development, and reduced neuroinflammation. However, none of the prior reviews presented a comprehensive correlation between physical activity, the endocannabinoid system, and neuropathologies.
Thus, our review provides a current state of knowledge of the endocannabinoid system, its action in physical activity, as well as neuropathologies and a possible correlation between all those fields. We believe that this might contribute to finding a new preventive and therapeutic approach to both neurological and neurodegenerative disorders.”
https://pubmed.ncbi.nlm.nih.gov/32545780/
https://www.mdpi.com/1422-0067/21/12/4221

 “The emergence of multi-drug resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) causes a major threat to public health due to its limited therapeutic options.
“The emergence of multi-drug resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) causes a major threat to public health due to its limited therapeutic options.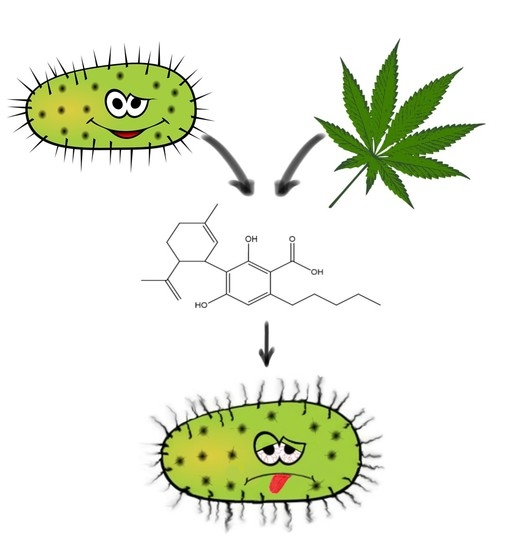
 “Irritable bowel syndrome (IBS) is a frequent cause of abdominal pain and altered bowel habits, which is associated with significant healthcare utilization.
“Irritable bowel syndrome (IBS) is a frequent cause of abdominal pain and altered bowel habits, which is associated with significant healthcare utilization. “Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
“Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
 “Identifying candidate drugs effective in the new coronavirus disease 2019 (Covid-19) is crucial,
“Identifying candidate drugs effective in the new coronavirus disease 2019 (Covid-19) is crucial,  “Cannabidiol (CBD) is a naturally occurring, non-psychotropic cannabinoid of the hemp plant Cannabis sativa L. and has been known to induce several physiological and pharmacological effects. While CBD is approved as a medicinal product subject to prescription, it is also widely sold over the counter (OTC) in the form of food supplements, cosmetics and electronic cigarette liquids. However, regulatory difficulties arise from its origin being a narcotic plant or its status as an unapproved novel food ingredient.
“Cannabidiol (CBD) is a naturally occurring, non-psychotropic cannabinoid of the hemp plant Cannabis sativa L. and has been known to induce several physiological and pharmacological effects. While CBD is approved as a medicinal product subject to prescription, it is also widely sold over the counter (OTC) in the form of food supplements, cosmetics and electronic cigarette liquids. However, regulatory difficulties arise from its origin being a narcotic plant or its status as an unapproved novel food ingredient. “Introduction: Severe Behavioural Problems (SBP) are a major contributor to morbidity in children with Intellectual Disability (ID). Medications used to treat SBP in ID are associated with a high risk of side effects. Cannabidiol has potential therapeutic effects in SBP. This pilot study aimed to investigate the feasibility of conducting a randomized placebo-controlled trial of cannabidiol to reduce SBP in children with ID.
“Introduction: Severe Behavioural Problems (SBP) are a major contributor to morbidity in children with Intellectual Disability (ID). Medications used to treat SBP in ID are associated with a high risk of side effects. Cannabidiol has potential therapeutic effects in SBP. This pilot study aimed to investigate the feasibility of conducting a randomized placebo-controlled trial of cannabidiol to reduce SBP in children with ID. “Background: Recent approved medicines whose active principles are Δ9Tetrahidrocannabinol (Δ9-THC) and/or cannabidiol (CBD) open novel perspectives for other phytocannabinoids also present in Cannabis sativa L. varieties. Furthermore, solid data on the potential benefits of acidic and varinic phytocannabinoids in a variety of diseases are already available. Mode of action of cannabigerol (CBG), cannabidiolic acid (CBDA), cannabigerolic acid (CBGA), cannabidivarin (CBDV) and cannabigerivarin (CBGV) is, to the very least, partial.
“Background: Recent approved medicines whose active principles are Δ9Tetrahidrocannabinol (Δ9-THC) and/or cannabidiol (CBD) open novel perspectives for other phytocannabinoids also present in Cannabis sativa L. varieties. Furthermore, solid data on the potential benefits of acidic and varinic phytocannabinoids in a variety of diseases are already available. Mode of action of cannabigerol (CBG), cannabidiolic acid (CBDA), cannabigerolic acid (CBGA), cannabidivarin (CBDV) and cannabigerivarin (CBGV) is, to the very least, partial.