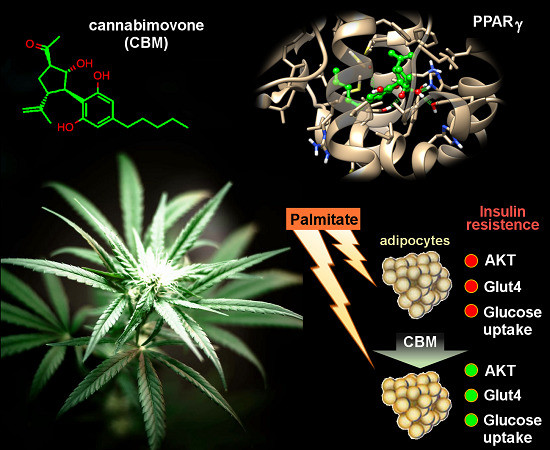
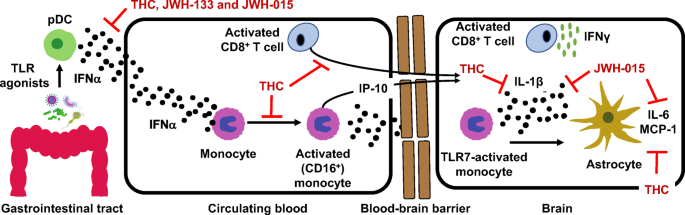
“HIV infection affects an estimated 38 million people. Approximately 50% of HIV patients exhibit neurocognitive dysfunction termed HIV-Associated Neurocognitive Disorder (HAND). HAND is a consequence of chronic low-level neuroinflammation due to HIV entry into the brain. Initially, monocytes become activated in circulation and traffic to the brain. Monocytes, when activated, become susceptible to infection by HIV and can then carry the virus across the blood brain barrier. Once in the brain, activated monocytes secrete chemokines, which recruit virus-specific CD8+ T cells into the brain to further promote neuroinflammation. HAND is closely linked to systemic inflammation driven, in part, by HIV but is also due to persistent translocation of microorganisms across the GI tract. Persistent anti-viral responses in the GI tract compromise microbial barrier integrity. Indeed, HIV patients can exhibit remarkably high levels of activated (CD16+) monocytes in circulation.
“HIV infection affects an estimated 38 million people. Approximately 50% of HIV patients exhibit neurocognitive dysfunction termed HIV-Associated Neurocognitive Disorder (HAND). HAND is a consequence of chronic low-level neuroinflammation due to HIV entry into the brain. Initially, monocytes become activated in circulation and traffic to the brain. Monocytes, when activated, become susceptible to infection by HIV and can then carry the virus across the blood brain barrier. Once in the brain, activated monocytes secrete chemokines, which recruit virus-specific CD8+ T cells into the brain to further promote neuroinflammation. HAND is closely linked to systemic inflammation driven, in part, by HIV but is also due to persistent translocation of microorganisms across the GI tract. Persistent anti-viral responses in the GI tract compromise microbial barrier integrity. Indeed, HIV patients can exhibit remarkably high levels of activated (CD16+) monocytes in circulation.
Recent studies, including our own, show that HIV patients using medical marijuana exhibit lower levels of circulating CD16+ monocytes than non-cannabis using HIV patients. Cannabis is a known immune modulator, including anti-inflammatory properties, mediated, in part, by ∆9-tetrahydrocannabinol (THC), as well as less characterized minor cannabinoids, such as cannabidiol (CBD), terpenes and presumably other cannabis constituents. The immune modulating activity of THC is largely mediated through cannabinoid receptors (CB) 1 and 2, with CB1 also responsible for the psychotropic properties of cannabis.
Here we discuss the anti-inflammatory properties of cannabinoids in the context of HIV and propose CB2 as a putative therapeutic target for the treatment of neuroinflammation. Graphical Abstract HIV-associated neurocognitive disorder is a systemic inflammatory disease leading to activation of plasmacytoid dendritic cells, monocytes and T cells. Monocyte and CD8 T cell migration across the BBB and interaction with astrocytes promotes neurotoxic inflammatory mediators release. CB2 ligands are proposed as therapeutics capable of suppressing systemic and localized inflammation.”
https://www.ncbi.nlm.nih.gov/pubmed/32409991
https://link.springer.com/article/10.1007%2Fs11481-020-09918-7

 “The antioxidant and CB2 receptor agonist properties of Δ9-tetrahydrocannabivarin (Δ9-THCV) afforded neuroprotection in experimental Parkinson’s disease (PD), whereas its CB1 receptor antagonist profile at doses lower than 5 mg/kg caused anti-hypokinetic effects.
“The antioxidant and CB2 receptor agonist properties of Δ9-tetrahydrocannabivarin (Δ9-THCV) afforded neuroprotection in experimental Parkinson’s disease (PD), whereas its CB1 receptor antagonist profile at doses lower than 5 mg/kg caused anti-hypokinetic effects. “Lenabasum is an oral synthetic
“Lenabasum is an oral synthetic  “Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and
“Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and  “The societal burden of ischemic stroke suggests a need for additional therapeutic categories in stroke prevention.
“The societal burden of ischemic stroke suggests a need for additional therapeutic categories in stroke prevention. “Both environmental and genetic factors are known to contribute to the development of anorexia nervosa (AN), but the exact etiology remains poorly understood.
“Both environmental and genetic factors are known to contribute to the development of anorexia nervosa (AN), but the exact etiology remains poorly understood. “Endocannabinoid system activity contributes to the homeostatic defense against aging and thus may counteract the progression of brain aging.
“Endocannabinoid system activity contributes to the homeostatic defense against aging and thus may counteract the progression of brain aging. “Neuroinflammation in the rostral ventrolateral medulla (RVLM) has been reported to be associated with hypertension. The upregulation and activation of the
“Neuroinflammation in the rostral ventrolateral medulla (RVLM) has been reported to be associated with hypertension. The upregulation and activation of the  “Medicinal use of
“Medicinal use of