 “Microglia, the resident immune cells of the central nervous system, mediate brain homeostasis by controlling neuronal proliferation/differentiation and synaptic activity. In response to external signals from neuropathological conditions, homeostatic (M0) microglia can adopt one of two activation states: the classical (M1) activation state, which secretes mediators of the proinflammatory response, and the alternative (M2) activation state, which presumably mediates the resolution of neuroinflammation and tissue repair/remodeling.
“Microglia, the resident immune cells of the central nervous system, mediate brain homeostasis by controlling neuronal proliferation/differentiation and synaptic activity. In response to external signals from neuropathological conditions, homeostatic (M0) microglia can adopt one of two activation states: the classical (M1) activation state, which secretes mediators of the proinflammatory response, and the alternative (M2) activation state, which presumably mediates the resolution of neuroinflammation and tissue repair/remodeling.
Since chronic inflammatory activation of microglia is correlated with several neurodegenerative diseases, functional modulation of microglial phenotypes has been considered as a potential therapeutic strategy.
The endocannabinoid (eCB) system, composed of cannabinoid receptors and ligands and their metabolic/biosynthetic enzymes, has been shown to activate anti-inflammatory signaling pathways that modulate immune cell functions. Growing evidence has demonstrated that endogenous, synthetic, and plant-derived eCB agonists possess therapeutic effects on several neuropathologies; however, the molecular mechanisms that mediate the anti-inflammatory effects have not yet been identified.
Over the last decade, it has been revealed that the eCB system modulates microglial activation and population. In this review, we thoroughly examine recent studies on microglial phenotype modulation by eCB in neuroinflammatory and neurodegenerative disease conditions.
We hypothesize that cannabinoid 2 receptor (CB2R) signaling shifts the balance of expression between neuroinflammatory (M1-type) genes, neuroprotective (M2-type) genes, and homeostatic (M0-type) genes toward the latter two gene expressions, by which microglia acquire therapeutic functionality.”
https://www.ncbi.nlm.nih.gov/pubmed/32117037
https://www.frontiersin.org/articles/10.3389/fneur.2020.00087/full

 “Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).
“Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).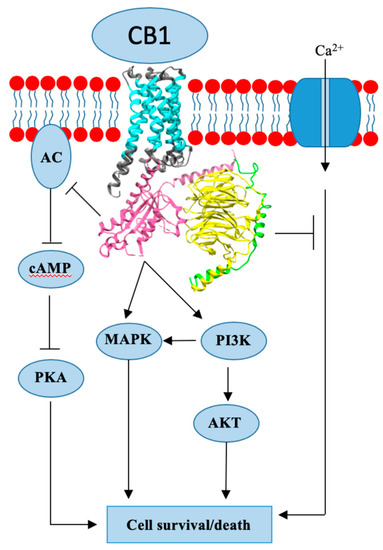
 “Herein, 11 general types of natural cannabinoids from Cannabis sativa as well as 50 (-)-CBD analogues with therapeutic potential were described. The underlying molecular mechanisms of CBD as a therapeutic candidate for epilepsy and neurodegenerative diseases were comprehensively clarified. CBD indirectly acts as an endogenous cannabinoid receptor agonist to exert its neuroprotective effects. CBD also promotes neuroprotection through different signal transduction pathways mediated indirectly by cannabinoid receptors. Furthermore, CBD prevents the glycogen synthase kinase 3β (GSK-3β) hyperphosphorylation caused by Aβ and may be developed as a new therapeutic candidate for Alzheimer’s disease.”
“Herein, 11 general types of natural cannabinoids from Cannabis sativa as well as 50 (-)-CBD analogues with therapeutic potential were described. The underlying molecular mechanisms of CBD as a therapeutic candidate for epilepsy and neurodegenerative diseases were comprehensively clarified. CBD indirectly acts as an endogenous cannabinoid receptor agonist to exert its neuroprotective effects. CBD also promotes neuroprotection through different signal transduction pathways mediated indirectly by cannabinoid receptors. Furthermore, CBD prevents the glycogen synthase kinase 3β (GSK-3β) hyperphosphorylation caused by Aβ and may be developed as a new therapeutic candidate for Alzheimer’s disease.”
 “Inflammatory Bowel Disease (IBD) is idiopathic, chronic and affects the gastrointestinal tract. It results from the association of genetic, environmental and immune deregulation, which culminates in the development and progression of the inflammatory process. In an attempt to reverse colonic inflammation, endogenous systems involved in intestinal physiology are studied and the cholinergic system is fundamental for this process. In addition, this system has anti-inflammatory action in experimental models of IBD. Another important endogenous system in regulating the exacerbated inflammatory response in the gut is mediated by endocannabinoids, which play an important role in restoring bowel functionality after the onset of the inflammatory process. There are several reports in the literature showing the interconnection between the cannabinoid and cholinergic systems in different tissues. Considering that the activation of the cholinergic system stimulates the production of cannabinoid agonists in the intestine, our hypothesis is that the interaction between the muscarinic system and the cannabinoid in the control of intestinal inflammation is mediated by endogenous cannabinoids, since they are stimulated by the activation of muscarinic receptors.”
“Inflammatory Bowel Disease (IBD) is idiopathic, chronic and affects the gastrointestinal tract. It results from the association of genetic, environmental and immune deregulation, which culminates in the development and progression of the inflammatory process. In an attempt to reverse colonic inflammation, endogenous systems involved in intestinal physiology are studied and the cholinergic system is fundamental for this process. In addition, this system has anti-inflammatory action in experimental models of IBD. Another important endogenous system in regulating the exacerbated inflammatory response in the gut is mediated by endocannabinoids, which play an important role in restoring bowel functionality after the onset of the inflammatory process. There are several reports in the literature showing the interconnection between the cannabinoid and cholinergic systems in different tissues. Considering that the activation of the cholinergic system stimulates the production of cannabinoid agonists in the intestine, our hypothesis is that the interaction between the muscarinic system and the cannabinoid in the control of intestinal inflammation is mediated by endogenous cannabinoids, since they are stimulated by the activation of muscarinic receptors.” “In the process of neonatal encephalopathy, oxidative stress and neuroinflammation have a prominent role after perinatal asphyxia. With the exception of therapeutic hypothermia, no therapeutic interventions are available in the clinical setting to target either the oxidative stress or inflammation, despite the high prevalence of neurological sequelae of this devastating condition.
“In the process of neonatal encephalopathy, oxidative stress and neuroinflammation have a prominent role after perinatal asphyxia. With the exception of therapeutic hypothermia, no therapeutic interventions are available in the clinical setting to target either the oxidative stress or inflammation, despite the high prevalence of neurological sequelae of this devastating condition. “Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g.,
“Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g.,  “Septic lung injury is one of main causes of high mortality in severe patients. Inhibition of excessive inflammatory response is considered as an effective strategy for septic lung injury.
“Septic lung injury is one of main causes of high mortality in severe patients. Inhibition of excessive inflammatory response is considered as an effective strategy for septic lung injury. “Human adipose tissue includes large quantities of mesenchymal stromal cells (atMSCs), which represent an abundant cell source for therapeutic applications in the field of regenerative medicine.
“Human adipose tissue includes large quantities of mesenchymal stromal cells (atMSCs), which represent an abundant cell source for therapeutic applications in the field of regenerative medicine.
 “Anticholinergic organophosphate (OP) agents act on the diverse serine hydrolases, thereby revealing unexpected biological effects. Epidemiological studies indicate a relationship between OP exposure and development of attention-deficit/hyperactivity disorder (ADHD)-like symptoms, whereas no plausible mechanism for the OP-induced ADHD has been established.
“Anticholinergic organophosphate (OP) agents act on the diverse serine hydrolases, thereby revealing unexpected biological effects. Epidemiological studies indicate a relationship between OP exposure and development of attention-deficit/hyperactivity disorder (ADHD)-like symptoms, whereas no plausible mechanism for the OP-induced ADHD has been established.