
“Δ9-THC suppresses cisplatin-induced vomiting through activation of cannabinoid CB1 receptors.
Cisplatin-evoked emesis is predominantly due to release of serotonin and substance P (SP) in the gut and the brainstem which subsequently stimulate their corresponding 5-HT3-and neurokinin NK1-receptors to induce vomiting. Δ9-THC can inhibit vomiting caused either by the serotonin precursor 5-HTP, or the 5-HT3 receptor selective agonist, 2-methyserotonin.
In the current study, we explored whether Δ9-THC and related CB1/CB2 receptor agonists (WIN55,212-2 and CP55,940) inhibit vomiting evoked by SP (50 mg/kg, i.p.) or the NK1 receptor selective agonist GR73632 (5 mg/kg, i.p.). Behavioral methods were employed to determine the antiemetic efficacy of cannabinoids in least shrews.
Our results showed that administration of varying doses of Δ9-THC (i.p. or s.c.), WIN55,212-2 (i.p.), or CP55,940 (i.p.) caused significant suppression of SP-evoked vomiting in a dose-dependent manner. When tested against GR73632, Δ9-THC also dose-dependently reduced the evoked emesis.
The antiemetic effect of Δ9-THC against SP-induced vomiting was prevented by low non-emetic doses of the CB1 receptor inverse-agonist/antagonist SR141716A (<10 mg/kg). We also found that the NK1 receptor antagonist netupitant can significantly suppress vomiting caused by a large emetic dose of SR141716A (20 mg/kg).
In sum, Δ9-THC and related cannabinoids suppress vomiting evoked by the nonselective (SP) and selective (GR73632) neurokinin NK1 receptor agonists via stimulation of cannabinoid CB1 receptors.”
https://www.ncbi.nlm.nih.gov/pubmed/31738934
https://www.sciencedirect.com/science/article/pii/S0014299919307587?via%3Dihub

 “L-dopa induced dyskinesia (LID) is a debilitating side-effect of the primary treatment used in Parkinson’s disease (PD), l-dopa. Here we investigate the effect of HU-308, a
“L-dopa induced dyskinesia (LID) is a debilitating side-effect of the primary treatment used in Parkinson’s disease (PD), l-dopa. Here we investigate the effect of HU-308, a  “The CB1 receptor mediates the central nervous system response to cannabinoids, and is a drug target for pain, anxiety and seizures.
“The CB1 receptor mediates the central nervous system response to cannabinoids, and is a drug target for pain, anxiety and seizures. “Bone cancer pain (BCP) is a severe complication of advanced bone cancer.
“Bone cancer pain (BCP) is a severe complication of advanced bone cancer. “WIN55,212-2 (WIN) is a synthetic agonist of
“WIN55,212-2 (WIN) is a synthetic agonist of 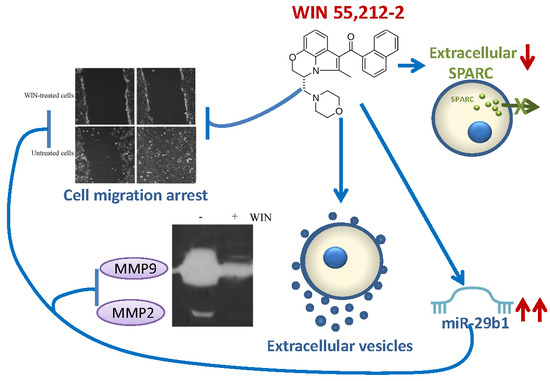
 “Although a lot of information can be found on the specific dual role of the endocannabinoid system in the emotional-related responses, little is known whether stimulation or inhibition of the CB receptors may affect the activity of the frequently prescribed antidepressant drugs.
“Although a lot of information can be found on the specific dual role of the endocannabinoid system in the emotional-related responses, little is known whether stimulation or inhibition of the CB receptors may affect the activity of the frequently prescribed antidepressant drugs.
 “Compounds present in Cannabis sativa such as phytocannabinoids and terpenoids may act in concert to elicit therapeutic effects.
“Compounds present in Cannabis sativa such as phytocannabinoids and terpenoids may act in concert to elicit therapeutic effects.  “Cannabinoid receptors have been detected in human gliomas and cannabinoids have been proposed as novel drug candidates in the treatment of brain tumors.
“Cannabinoid receptors have been detected in human gliomas and cannabinoids have been proposed as novel drug candidates in the treatment of brain tumors.