 “Cannabidiol (CBD) is a non-intoxicating cannabinoid derived from Cannabis sativa. CBD initially drew scientific interest due to its anticonvulsant properties but increasing evidence of other therapeutic effects has attracted the attention of additional clinical and non-clinical populations, including athletes.
“Cannabidiol (CBD) is a non-intoxicating cannabinoid derived from Cannabis sativa. CBD initially drew scientific interest due to its anticonvulsant properties but increasing evidence of other therapeutic effects has attracted the attention of additional clinical and non-clinical populations, including athletes.
Unlike the intoxicating cannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC), CBD is no longer prohibited by the World Anti-Doping Agency and appears to be safe and well-tolerated in humans. It has also become readily available in many countries with the introduction of over-the-counter “nutraceutical” products.
The aim of this narrative review was to explore various physiological and psychological effects of CBD that may be relevant to the sport and/or exercise context and to identify key areas for future research. As direct studies of CBD and sports performance are is currently lacking, evidence for this narrative review was sourced from preclinical studies and a limited number of clinical trials in non-athlete populations.
Preclinical studies have observed robust anti-inflammatory, neuroprotective and analgesic effects of CBD in animal models. Preliminary preclinical evidence also suggests that CBD may protect against gastrointestinal damage associated with inflammation and promote healing of traumatic skeletal injuries. However, further research is required to confirm these observations.
Early stage clinical studies suggest that CBD may be anxiolytic in “stress-inducing” situations and in individuals with anxiety disorders. While some case reports indicate that CBD improves sleep, robust evidence is currently lacking. Cognitive function and thermoregulation appear to be unaffected by CBD while effects on food intake, metabolic function, cardiovascular function, and infection require further study.
CBD may exert a number of physiological, biochemical, and psychological effects with the potential to benefit athletes. However, well controlled, studies in athlete populations are required before definitive conclusions can be reached regarding the utility of CBD in supporting athletic performance.”
https://pubmed.ncbi.nlm.nih.gov/32632671/
“CBD has been reported to exert a number of physiological, biochemical, and psychological effects that have the potential to benefit athletes. For instance, there is preliminary supportive evidence for anti-inflammatory, neuroprotective, analgesic, and anxiolytic actions of CBD and the possibility it may protect against GI damage associated with inflammation and promote the healing of traumatic skeletal injuries.”
https://sportsmedicine-open.springeropen.com/articles/10.1186/s40798-020-00251-0
 “Cholesterol plays vital roles in many central physiological and pathological processes. As a key component in the cell membrane, cholesterol can regulate a variety of ion channels, including ligand-gated ion channels (LGICs). However, relatively little is known about the molecular detail and in vivo consequence of cholesterol-LGIC interaction. Here, we reveal that membrane cholesterol depletion significantly inhibits the potentiating effects of dehydroxylcannabidiol (DH-CBD) on glycine-activated currents (IGly) in HEK 293T cells expressing α1/α3 glycine receptors (GlyRs). Simvastatin considerably decreases cholesterol levels and DH-CBD-induced potentiation of IGly in the spinal cord of mice. Simvastatin also significantly decreases DH-CBD analgesia in acute and chronic pain of mice. The cholesterol levels in the dorsal horn of spinal cord, measured by mass spectrometry imaging, are specifically correlated with cannabinoid potentiation of spinal GlyRs and cannabinoid-induced analgesia. These findings suggest that spinal cholesterol is critical for the efficacy of glycinergic cannabinoid-induced analgesia.”
“Cholesterol plays vital roles in many central physiological and pathological processes. As a key component in the cell membrane, cholesterol can regulate a variety of ion channels, including ligand-gated ion channels (LGICs). However, relatively little is known about the molecular detail and in vivo consequence of cholesterol-LGIC interaction. Here, we reveal that membrane cholesterol depletion significantly inhibits the potentiating effects of dehydroxylcannabidiol (DH-CBD) on glycine-activated currents (IGly) in HEK 293T cells expressing α1/α3 glycine receptors (GlyRs). Simvastatin considerably decreases cholesterol levels and DH-CBD-induced potentiation of IGly in the spinal cord of mice. Simvastatin also significantly decreases DH-CBD analgesia in acute and chronic pain of mice. The cholesterol levels in the dorsal horn of spinal cord, measured by mass spectrometry imaging, are specifically correlated with cannabinoid potentiation of spinal GlyRs and cannabinoid-induced analgesia. These findings suggest that spinal cholesterol is critical for the efficacy of glycinergic cannabinoid-induced analgesia.”
 “Cannabinoid CB2 receptor (CB2) agonists are potential analgesics void of psychotropic effects.
“Cannabinoid CB2 receptor (CB2) agonists are potential analgesics void of psychotropic effects. “Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
“Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.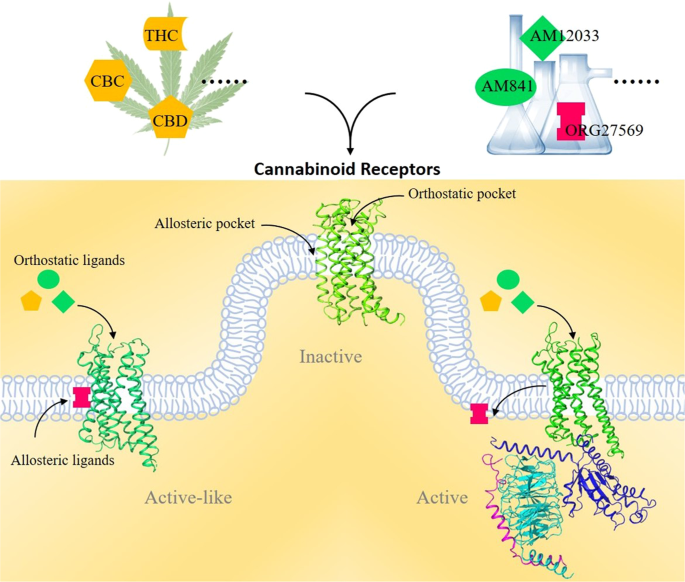
 “Self-reported cannabis use has increased since its recent legalization in many states.
“Self-reported cannabis use has increased since its recent legalization in many states. “Cannabidiol (CBD) is a non-intoxicating cannabinoid derived from Cannabis sativa. CBD initially drew scientific interest due to its anticonvulsant properties but increasing evidence of other therapeutic effects has attracted the attention of additional clinical and non-clinical populations, including athletes.
“Cannabidiol (CBD) is a non-intoxicating cannabinoid derived from Cannabis sativa. CBD initially drew scientific interest due to its anticonvulsant properties but increasing evidence of other therapeutic effects has attracted the attention of additional clinical and non-clinical populations, including athletes. “Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes.
“Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes. “Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
“Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
 “Painful tonic spasm (PTS) is a common yet debilitating symptom in patients with neuromyelitis optica spectrum disorder (NMOSD), especially those with longitudinally extensive transverse myelitis. Although carbamazepine is an effective treatment, it poses the risk of severe adverse reactions, such as Steven-Johnson syndrome (SJS).
“Painful tonic spasm (PTS) is a common yet debilitating symptom in patients with neuromyelitis optica spectrum disorder (NMOSD), especially those with longitudinally extensive transverse myelitis. Although carbamazepine is an effective treatment, it poses the risk of severe adverse reactions, such as Steven-Johnson syndrome (SJS).