 “Chronic pain is a common complaint among patients, and rheumatic diseases are a common cause for chronic pain. Current pharmacological interventions for chronic pain are not always useful or safe enough for long-term use.
“Chronic pain is a common complaint among patients, and rheumatic diseases are a common cause for chronic pain. Current pharmacological interventions for chronic pain are not always useful or safe enough for long-term use.
Cannabis and cannabinoids are currently being studied due to their potential as analgesics. In this review we will discuss current literature regarding cannabinoids and cannabis as treatment for rheumatic diseases.
Fibromyalgia is a prevalent rheumatic disease that causes diffuse pain, fatigue, and sleep disturbances. Treatment of this syndrome is symptomatic, and it has been suggested that cannabis and cannabinoids could potentially alleviate some of the symptoms associated with fibromyalgia. In this review we cite some of the evidence that supports this claim. However, data on long-term efficacy and safety of cannabinoid and cannabis use are still lacking.
Cannabinoids and cannabis are commonly investigated as analgesic agents, but in recent years more evidence has accumulated on their potential immune-modulatory effect, supported by results in animal models of certain rheumatic diseases. While results that demonstrate the same effect in humans are still lacking, cannabinoids and cannabis remain potential drugs to alleviate the pain associated with rheumatic diseases, as they were shown to be safe and to cause limited adverse effects.”

 “Critically ill patients with sepsis require a multidisciplinary approach, as this situation implies multiorgan distress, with most of the bodily biochemical and cellular systems being affected by the condition. Moreover, sepsis is characterized by a multitude of biochemical interactions and by dynamic changes of the immune system. At the moment, there is a gap in our understanding of the cellular, genetic, and molecular mechanisms involved in sepsis.
“Critically ill patients with sepsis require a multidisciplinary approach, as this situation implies multiorgan distress, with most of the bodily biochemical and cellular systems being affected by the condition. Moreover, sepsis is characterized by a multitude of biochemical interactions and by dynamic changes of the immune system. At the moment, there is a gap in our understanding of the cellular, genetic, and molecular mechanisms involved in sepsis.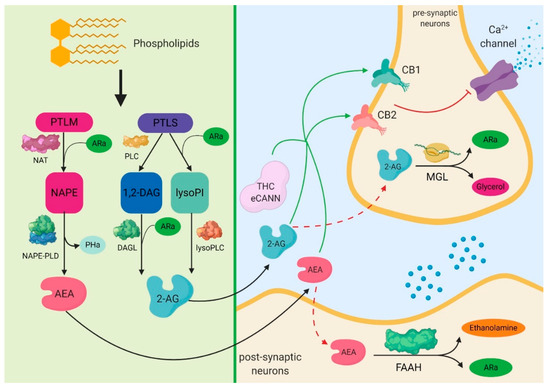
 “Human adipose tissue includes large quantities of mesenchymal stromal cells (atMSCs), which represent an abundant cell source for therapeutic applications in the field of regenerative medicine.
“Human adipose tissue includes large quantities of mesenchymal stromal cells (atMSCs), which represent an abundant cell source for therapeutic applications in the field of regenerative medicine.
 “Renal ischemia-reperfusion injury (IRI) is a major cause of acute kidney injury (AKI) and even induces remote organ damage.
“Renal ischemia-reperfusion injury (IRI) is a major cause of acute kidney injury (AKI) and even induces remote organ damage. “Drugs selectively targeting CB2 hold promise for treating neurodegenerative disorders, inflammation, and pain while avoiding psychotropic side effects mediated by CB1. The mechanisms underlying CB2 activation and signaling are poorly understood but critical for drug design. Here we report the cryo-EM structure of the human CB2-Gi signaling complex bound to the agonist WIN 55,212-2. The 3D structure reveals the binding mode of WIN 55,212-2 and structural determinants for distinguishing CB2 agonists from antagonists, which are supported by a pair of rationally designed agonist and antagonist. Further structural analyses with computational docking results uncover the differences between CB2 and CB1 in receptor activation, ligand recognition, and Gi coupling. These findings are expected to facilitate rational structure-based discovery of drugs targeting the
“Drugs selectively targeting CB2 hold promise for treating neurodegenerative disorders, inflammation, and pain while avoiding psychotropic side effects mediated by CB1. The mechanisms underlying CB2 activation and signaling are poorly understood but critical for drug design. Here we report the cryo-EM structure of the human CB2-Gi signaling complex bound to the agonist WIN 55,212-2. The 3D structure reveals the binding mode of WIN 55,212-2 and structural determinants for distinguishing CB2 agonists from antagonists, which are supported by a pair of rationally designed agonist and antagonist. Further structural analyses with computational docking results uncover the differences between CB2 and CB1 in receptor activation, ligand recognition, and Gi coupling. These findings are expected to facilitate rational structure-based discovery of drugs targeting the  “The legalization of
“The legalization of  “Medical marijuana is becoming widely available to patients in the U.S. and with recreational marijuana now legalized in many states, patient interest is on the rise.
“Medical marijuana is becoming widely available to patients in the U.S. and with recreational marijuana now legalized in many states, patient interest is on the rise. “Palliative medicine physicians are challenged by lack of guidance regarding effectiveness and dosing of cannabis products in the setting of their emerging popularity.
“Palliative medicine physicians are challenged by lack of guidance regarding effectiveness and dosing of cannabis products in the setting of their emerging popularity. “Abnormal
“Abnormal