“The main aspects of severe COVID-19 disease pathogenesis include hyper-induction of proinflammatory cytokines, also known as ‘cytokine storm’, that precedes acute respiratory distress syndrome (ARDS) and often leads to death. COVID-19 patients often suffer from lung fibrosis, a serious and untreatable condition. There remains no effective treatment for these complications.
“The main aspects of severe COVID-19 disease pathogenesis include hyper-induction of proinflammatory cytokines, also known as ‘cytokine storm’, that precedes acute respiratory distress syndrome (ARDS) and often leads to death. COVID-19 patients often suffer from lung fibrosis, a serious and untreatable condition. There remains no effective treatment for these complications.
Out of all cytokines, TNFα and IL-6 play crucial roles in cytokine storm pathogenesis and are likely responsible for the escalation in disease severity. These cytokines also partake in the molecular pathogenesis of fibrosis. Therefore, new approaches are urgently needed, that can efficiently and swiftly downregulate TNFα, IL-6, and the inflammatory cytokine cascade, in order to curb inflammation and prevent fibrosis, and lead to disease remission.
Cannabis sativa has been proposed to modulate gene expression and inflammation and is under investigation for several potential therapeutic applications against autoinflammatory diseases and cancer. Here, we hypothesized that the extracts of novel C. sativa cultivars may be used to downregulate the expression of pro-inflammatory cytokines and pathways involved in inflammation and fibrosis.
Novel anti-TNFα and anti-IL-6 cannabis extracts can be useful additions to the current anti-inflammatory regimens to treat COVID-19, as well as various rheumatological diseases and conditions, and ‘inflammaging’ – the inflammatory underpinning of aging and frailty.”

 “Inflammasomes are cytoplasmic inflammatory signaling protein complexes that detect microbial materials, sterile inflammatory insults, and certain host-derived elements. Inflammasomes, once activated, promote caspase-1-mediated maturation and secretion of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18, leading to pyroptosis. Current advances in inflammasome research support their involvement in the development of chronic inflammatory disorders in contrast to their role in regulating innate immunity.
“Inflammasomes are cytoplasmic inflammatory signaling protein complexes that detect microbial materials, sterile inflammatory insults, and certain host-derived elements. Inflammasomes, once activated, promote caspase-1-mediated maturation and secretion of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18, leading to pyroptosis. Current advances in inflammasome research support their involvement in the development of chronic inflammatory disorders in contrast to their role in regulating innate immunity. “Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.
“Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.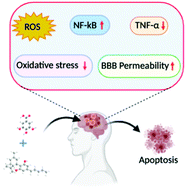
 “Considering the advantages of using medicinal herbs as supplementary treatments to sensitize conventional anti-cancer drugs, studying functional mechanisms and regulatory effects of Echinacea purpurea (as a non-cannabinoid plant)
“Considering the advantages of using medicinal herbs as supplementary treatments to sensitize conventional anti-cancer drugs, studying functional mechanisms and regulatory effects of Echinacea purpurea (as a non-cannabinoid plant) 



 “Cannabis sativa L. is an aromatic annual herb belonging to the family Cannabaceae and it is widely distributed worldwide. Cultivation, selling, and consumption of cannabis and cannabis related products, regardless of its use, was prohibited in Lebanon until April 22, 2020. Nevertheless, cannabis oil has been traditionally used unlawfully for many years in Lebanon to treat diseases such as arthritis, diabetes, cancer and few neurological disorders.
“Cannabis sativa L. is an aromatic annual herb belonging to the family Cannabaceae and it is widely distributed worldwide. Cultivation, selling, and consumption of cannabis and cannabis related products, regardless of its use, was prohibited in Lebanon until April 22, 2020. Nevertheless, cannabis oil has been traditionally used unlawfully for many years in Lebanon to treat diseases such as arthritis, diabetes, cancer and few neurological disorders.
 “Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder with a multifactorial etiology. Latest researches are raising the hypothesis of a link between the onset of the main behavioral symptoms of ASD and the chronic neuroinflammatory condition of the autistic brain; increasing evidence of this connection is shedding light on new possible players in the pathogenesis of ASD.
“Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder with a multifactorial etiology. Latest researches are raising the hypothesis of a link between the onset of the main behavioral symptoms of ASD and the chronic neuroinflammatory condition of the autistic brain; increasing evidence of this connection is shedding light on new possible players in the pathogenesis of ASD. “A significant number of cannabinoids are known to have analgesic and anti-inflammatory properties in various diseases. Due to their presynaptic/terminal location, cannabinoid receptors can inhibit synaptic transmission and have the potential to regulate neurogenic inflammation. Neurogenic inflammation occurs when a noxious signal is detected in the periphery initiating an antidromic axon reflex in the same sensory neurone leading to depolarization of the afferent terminal. Neuropeptides are subsequently released and contribute to vasodilation, plasma extravasation and modulation of immune cells. Endocannabinoids, synthetic cannabinoids and phytocannabinoids can reduce neuroinflammation by inhibiting afferent firing and inflammatory neuropeptide release. Thus, in addition to a direct effect on vascular smooth muscle and inflammatory cells, cannabinoids can reduce inflammation by silencing small diameter neurones. This review examines the neuropharmacological processes involved in regulating antidromic depolarization of afferent nerve terminals by cannabinoids and the control of neurogenic inflammation in different diseases.”
“A significant number of cannabinoids are known to have analgesic and anti-inflammatory properties in various diseases. Due to their presynaptic/terminal location, cannabinoid receptors can inhibit synaptic transmission and have the potential to regulate neurogenic inflammation. Neurogenic inflammation occurs when a noxious signal is detected in the periphery initiating an antidromic axon reflex in the same sensory neurone leading to depolarization of the afferent terminal. Neuropeptides are subsequently released and contribute to vasodilation, plasma extravasation and modulation of immune cells. Endocannabinoids, synthetic cannabinoids and phytocannabinoids can reduce neuroinflammation by inhibiting afferent firing and inflammatory neuropeptide release. Thus, in addition to a direct effect on vascular smooth muscle and inflammatory cells, cannabinoids can reduce inflammation by silencing small diameter neurones. This review examines the neuropharmacological processes involved in regulating antidromic depolarization of afferent nerve terminals by cannabinoids and the control of neurogenic inflammation in different diseases.” “Effective treatment choices to the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) are limited because of the absence of effective target-based therapeutics. The main object of the current research was to estimate the antiviral activity of cannabinoids (CBDs) against the human coronavirus SARS-CoV-2.
“Effective treatment choices to the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) are limited because of the absence of effective target-based therapeutics. The main object of the current research was to estimate the antiviral activity of cannabinoids (CBDs) against the human coronavirus SARS-CoV-2.
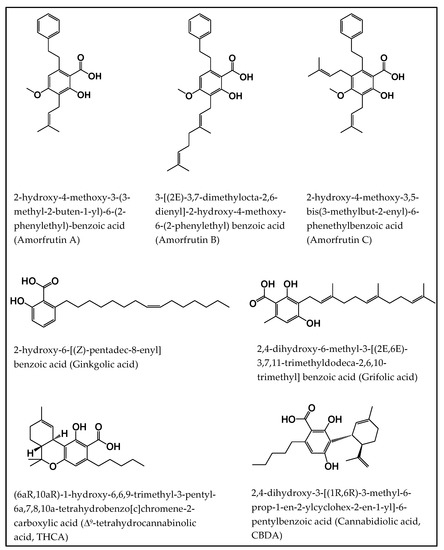
 “Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses. Increasing reports suggest that this phenolic compound and its derivatives, collectively termed salicylates, not only regulate plant defense but also have beneficial effects on human health. Both natural and synthetic salicylates are known to have multiple targets in humans, thereby exhibiting various appreciating pharmacological roles, including anti-inflammatory, anticancer, neuroprotective, antidiabetic effects, and so on. The role of some salicylates, such as acetylsalicylic acid (aspirin), 5-aminosalicylic acid (mesalazine), and amorfrutins in human diseases has been well studied in vitro. However, their clinical significance in different diseases is largely unknown. Based on recent studies, five natural salicylates, including amorfrutin, ginkgolic acid, grifolic acid, tetrahydrocannabinolic acid, and cannabidiolic acid, showed potential roles in different challenging human diseases. This review summarizes together some of the recent information on multitarget regulatory activities of these natural salicylates and their pharmacological roles in human health.”
“Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses. Increasing reports suggest that this phenolic compound and its derivatives, collectively termed salicylates, not only regulate plant defense but also have beneficial effects on human health. Both natural and synthetic salicylates are known to have multiple targets in humans, thereby exhibiting various appreciating pharmacological roles, including anti-inflammatory, anticancer, neuroprotective, antidiabetic effects, and so on. The role of some salicylates, such as acetylsalicylic acid (aspirin), 5-aminosalicylic acid (mesalazine), and amorfrutins in human diseases has been well studied in vitro. However, their clinical significance in different diseases is largely unknown. Based on recent studies, five natural salicylates, including amorfrutin, ginkgolic acid, grifolic acid, tetrahydrocannabinolic acid, and cannabidiolic acid, showed potential roles in different challenging human diseases. This review summarizes together some of the recent information on multitarget regulatory activities of these natural salicylates and their pharmacological roles in human health.”