“UV radiation is a well-established environmental risk factor known to cause oxidative stress and disrupt the metabolism of keratinocyte phospholipids. Cannabidiol (CBD) is a phytocannabinoid with anti-inflammatory and antioxidant effects.
“UV radiation is a well-established environmental risk factor known to cause oxidative stress and disrupt the metabolism of keratinocyte phospholipids. Cannabidiol (CBD) is a phytocannabinoid with anti-inflammatory and antioxidant effects.
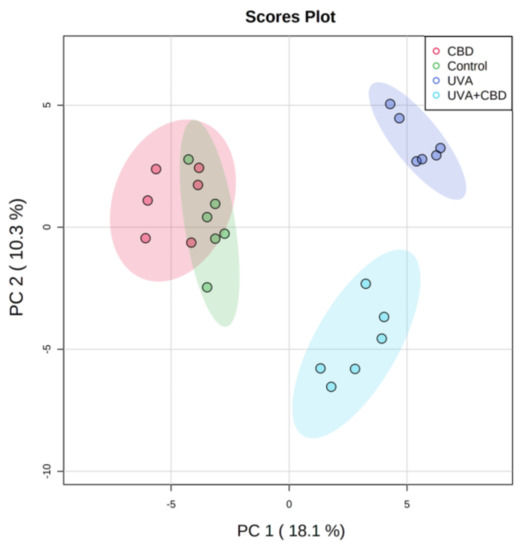
In this study, we examined changes in the keratinocyte phospholipid profile from nude rat skin exposed to UVA and UVB radiation that was also treated topically with CBD.
UVA and UVB radiation promoted up-regulation of phosphatidylcholines (PC), lysophosphatidylcholines (LPC), phosphatidylethanolamines (PE) and down-regulation of sphingomyelin (SM) levels and enhanced the activity of phospholipase A2 (PLA2) and sphingomyelinase (SMase).
Application of CBD to the skin of control rats led to down-regulation of SM and up-regulation of SMase activity. After CBD treatment of rats irradiated with UVA or UVB, SM was up-regulated and down-regulated, respectively, while ceramide (CER) levels and SMase activity were down-regulated and up-regulated, respectively. CBD applied to the skin of UV-irradiated rats down-regulated LPC, up-regulated PE and phosphatidylserines (PS) and reduced PLA2 activity.
In conclusion, up-regulation of PS may suggest that CBD inhibits their oxidative modification, while changes in the content of PE and SM may indicate a role of CBD in promoting autophagy and improving the status of the transepidermal barrier.”
https://pubmed.ncbi.nlm.nih.gov/33255796/
https://www.mdpi.com/2076-3921/9/12/1178

 “Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS.
“Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS. “With the current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality.
“With the current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality. “The COVID-19 pandemic caused by SARS-CoV-2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune-inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably.
“The COVID-19 pandemic caused by SARS-CoV-2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune-inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably.
 “The aberrant accumulation of disease-specific protein aggregates accompanying cognitive decline is a pathological hallmark of age-associated neurological disorders, also termed as proteinopathies, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis.
“The aberrant accumulation of disease-specific protein aggregates accompanying cognitive decline is a pathological hallmark of age-associated neurological disorders, also termed as proteinopathies, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis. “Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown.
“Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown. “(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants.
“(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants. “Multiple therapeutic properties have been attributed to Cannabis sativa. However, further research is required to unveil the medicinal potential of Cannabis and the relationship between biological activity and chemical profile.
“Multiple therapeutic properties have been attributed to Cannabis sativa. However, further research is required to unveil the medicinal potential of Cannabis and the relationship between biological activity and chemical profile.