
“New tetrahydrocannabinolic acid (THCA) derivatives ALAM027 and ALAM108 were proposed for the treatment of the pancreatic cancer disease.
Methods: The in vitro effect of new cannabinoids ALAM027 and ALAM108 was tested against PANC-1 and AsPC-1 cell lines by CellTiter Glo assay. Pancreatic cancer xenograft model was used for the in vivo anticancer activity study of these compounds on PANC-1 cells.
Results: The in vitro study of new cannabinoids showed greater activity of ALAM108 than ALAM027 both for PANC-1 and AsPC-1 cells. The in vivo study of new cannabinoids on PANC-1 cells showed that their oral administration was effective in reducing tumor volume and tumor weight, and did not lead to any discomfort and weight loss of mice.
Conclusion: The cannabinoids ALAM108 and ALAM027 inhibited the tumor growing 1.6-2 times in mice with human PANC-1 cells.”
https://pubmed.ncbi.nlm.nih.gov/32642629/
“The in vitro study of new cannabinoids showed greater activity of ALAM108 than of ALAM027 both for PANC-1 and AsPC-1 pancreas tumor cells. The in vivo study of these cannabinoids on PANC-1 cells showed that their oral administration decreased the tumor size 1.6–2 times and did not lead to any discomfort, psychotic effects, and weight loss of mice. Further study of these compounds will allow to determine the mechanism of their action on cancer cells and may open the way to new therapeutic drugs based on THCA.”
https://www.liebertpub.com/doi/10.1089/pancan.2020.0003


 “The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
“The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.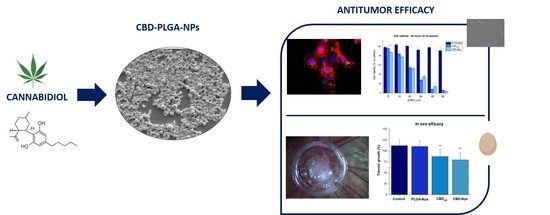
 “Cannabis has been used to relieve the symptoms of disease for thousands of years. However, social and political biases have limited effective interrogation of the potential benefits of cannabis and polarised public opinion.
“Cannabis has been used to relieve the symptoms of disease for thousands of years. However, social and political biases have limited effective interrogation of the potential benefits of cannabis and polarised public opinion. “Colorectal cancer (CRC) has a high mortality rate and is one of the most difficult diseases to manage due to tumour resistance and metastasis. The treatment of choice for CRC is reliant on the phase and time of diagnosis. Despite several conventional treatments available to treat CRC (surgical excision, chemo-, radiation- and immune-therapy), resistance is a major challenge, especially if it has metastasized. Additionally, these treatments often cause unwanted adverse side effects and so it remains imperative to investigate, alternative combination therapies.
“Colorectal cancer (CRC) has a high mortality rate and is one of the most difficult diseases to manage due to tumour resistance and metastasis. The treatment of choice for CRC is reliant on the phase and time of diagnosis. Despite several conventional treatments available to treat CRC (surgical excision, chemo-, radiation- and immune-therapy), resistance is a major challenge, especially if it has metastasized. Additionally, these treatments often cause unwanted adverse side effects and so it remains imperative to investigate, alternative combination therapies. “Cannabis sativa produces hundreds of phytocannabinoids and terpenes.
“Cannabis sativa produces hundreds of phytocannabinoids and terpenes. “Exemestane is one of the aromatase inhibitors (AI) used as first line treatment for estrogen-receptor positive breast cancer in post-menopausal women. Exemestane acts by inhibiting aromatase, the enzyme responsible for the conversion of androgens to estrogens and also by promoting apoptosis of breast cancer cells. Nevertheless, despite its therapeutic success, this AI, after prolonged treatment, can induce acquired resistance, which causes tumor relapse. Therefore, it is important to find new strategies to overcome resistance in order to improve breast cancer treatment.
“Exemestane is one of the aromatase inhibitors (AI) used as first line treatment for estrogen-receptor positive breast cancer in post-menopausal women. Exemestane acts by inhibiting aromatase, the enzyme responsible for the conversion of androgens to estrogens and also by promoting apoptosis of breast cancer cells. Nevertheless, despite its therapeutic success, this AI, after prolonged treatment, can induce acquired resistance, which causes tumor relapse. Therefore, it is important to find new strategies to overcome resistance in order to improve breast cancer treatment. “The recent announcement of marijuana legalization in Canada spiked many discussions about potential health benefits of Cannabis sativa.
“The recent announcement of marijuana legalization in Canada spiked many discussions about potential health benefits of Cannabis sativa.  “Over the last decades a renewed interest in n-3 very long polyunsaturated fatty acids (PUFAs), derived mainly from fish oils in the human diet, has been observed because of their potential effects against cancer diseases, including breast carcinoma. These n-3 PUFAs mainly consist of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that, alone or in combination with anticancer agents, induce cell cycle arrest, autophagy, apoptosis, and tumor growth inhibition. A large number of molecular targets of n-3 PUFAs have been identified and multiple mechanisms appear to underlie their antineoplastic activities. Evidence exists that EPA and DHA also elicit anticancer effects by the conversion to their corresponding ethanolamide derivatives in cancer cells, by binding and activation of different receptors and distinct signaling pathways. Other conjugates with serotonin or dopamine have been found to exert anti-inflammatory activities in breast tumor microenvironment, indicating the importance of these compounds as modulators of tumor epithelial/stroma interplay. The objective of this review is to provide a general overview and an update of the current n-3 PUFA derivative research and to highlight intriguing aspects of the potential therapeutic benefits of these low-toxicity compounds in breast cancer treatment and care.”
“Over the last decades a renewed interest in n-3 very long polyunsaturated fatty acids (PUFAs), derived mainly from fish oils in the human diet, has been observed because of their potential effects against cancer diseases, including breast carcinoma. These n-3 PUFAs mainly consist of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that, alone or in combination with anticancer agents, induce cell cycle arrest, autophagy, apoptosis, and tumor growth inhibition. A large number of molecular targets of n-3 PUFAs have been identified and multiple mechanisms appear to underlie their antineoplastic activities. Evidence exists that EPA and DHA also elicit anticancer effects by the conversion to their corresponding ethanolamide derivatives in cancer cells, by binding and activation of different receptors and distinct signaling pathways. Other conjugates with serotonin or dopamine have been found to exert anti-inflammatory activities in breast tumor microenvironment, indicating the importance of these compounds as modulators of tumor epithelial/stroma interplay. The objective of this review is to provide a general overview and an update of the current n-3 PUFA derivative research and to highlight intriguing aspects of the potential therapeutic benefits of these low-toxicity compounds in breast cancer treatment and care.” “The endocannabinoid system (ECS) comprises the canonical receptor subtypes CB1R and CB2R and endocannabinoids (anandamide, AEA and 2-arachidonoylglycerol, 2-AG), and a “non-canonical” extended signaling network consisting of: (i) other fatty acid derivatives; (ii) the defined “ionotropic cannabinoid receptors” (TRP channels); other GPCRs (GPR55, PPARα); (iii) enzymes involved in the biosynthesis and degradation of endocannabinoids (FAAH and MAGL); and (iv) protein transporters (FABP family).The ECS is currently a hot topic due to its involvement in cancer and pain.
“The endocannabinoid system (ECS) comprises the canonical receptor subtypes CB1R and CB2R and endocannabinoids (anandamide, AEA and 2-arachidonoylglycerol, 2-AG), and a “non-canonical” extended signaling network consisting of: (i) other fatty acid derivatives; (ii) the defined “ionotropic cannabinoid receptors” (TRP channels); other GPCRs (GPR55, PPARα); (iii) enzymes involved in the biosynthesis and degradation of endocannabinoids (FAAH and MAGL); and (iv) protein transporters (FABP family).The ECS is currently a hot topic due to its involvement in cancer and pain. “Novel anticancer medicines, including targeted therapies and immune checkpoint inhibitors, have greatly improved the management of cancers. However, both conventional and new anticancer treatments induce cardiac adverse effects, which remain a critical issue in clinic.
“Novel anticancer medicines, including targeted therapies and immune checkpoint inhibitors, have greatly improved the management of cancers. However, both conventional and new anticancer treatments induce cardiac adverse effects, which remain a critical issue in clinic.