 “We used mouse microglial cells in culture activated by lipopolysaccharide (LPS, 10 ng/ml) to study the anti-inflammatory potential of cannabidiol (CBD), the major nonpsychoactive component of cannabis.
“We used mouse microglial cells in culture activated by lipopolysaccharide (LPS, 10 ng/ml) to study the anti-inflammatory potential of cannabidiol (CBD), the major nonpsychoactive component of cannabis.
Under LPS stimulation, CBD (1-10 μM) potently inhibited the release of prototypical proinflammatory cytokines (TNF-α and IL-1β) and that of glutamate, a noncytokine mediator of inflammation. The effects of CBD were predominantly receptor-independent and only marginally blunted by blockade of CB2 receptors.
We established that CBD inhibited a mechanism involving, sequentially, NADPH oxidase-mediated ROS production and NF-κB-dependent signaling events. In line with these observations, active concentrations of CBD demonstrated an intrinsic free-radical scavenging capacity in the cell-free DPPH assay.
Of interest, CBD also prevented the rise in glucose uptake observed in microglial cells challenged with LPS, as did the inhibitor of NADPH oxidase apocynin and the inhibitor of IκB kinase-2, TPCA-1. This indicated that the capacity of CBD to prevent glucose uptake also contributed to its anti-inflammatory activity.
Supporting this view, the glycolytic inhibitor 2-deoxy-d-glucose (2-DG) mimicked the antioxidant/immunosuppressive effects of CBD. Interestingly, CBD and 2-DG, as well as apocynin and TPCA-1 caused a reduction in glucose-derived NADPH, a cofactor required for NADPH oxidase activation and ROS generation.
These different observations suggest that CBD exerts its anti-inflammatory effects towards microglia through an intrinsic antioxidant effect, which is amplified through inhibition of glucose-dependent NADPH synthesis.
These results also further confirm that CBD may have therapeutic utility in conditions where neuroinflammatory processes are prominent.”
https://www.ncbi.nlm.nih.gov/pubmed/31647138
https://onlinelibrary.wiley.com/doi/abs/10.1002/glia.23738
 “Chronic cerebral hypoperfusion (CCH) is a major contributor to cognitive decline and degenerative processes leading to Alzheimer’s disease, vascular dementia, and aging. However, the delicate mechanism of CCH-induced neuronal damage, and therefore proper treatment, remains unclear.
“Chronic cerebral hypoperfusion (CCH) is a major contributor to cognitive decline and degenerative processes leading to Alzheimer’s disease, vascular dementia, and aging. However, the delicate mechanism of CCH-induced neuronal damage, and therefore proper treatment, remains unclear.
 “Hepatorenal syndrome (HRS) is a life-threatening complication of end-stage liver disease characterized by the rapid decline of kidney function. Herein, we explored the therapeutic potential of targeting the
“Hepatorenal syndrome (HRS) is a life-threatening complication of end-stage liver disease characterized by the rapid decline of kidney function. Herein, we explored the therapeutic potential of targeting the 
 “Neuroinflammation is associated with many neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS). In this study, we investigate the anti-inflammatory, anti-oxidant, and anti-apoptotic properties of two non-psychoactive phytocannabinoids, cannabigerol (CBG) and
“Neuroinflammation is associated with many neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS). In this study, we investigate the anti-inflammatory, anti-oxidant, and anti-apoptotic properties of two non-psychoactive phytocannabinoids, cannabigerol (CBG) and  “Cannabis sativa L. is one of the most-studied species for its phytochemistry due to the abundance of secondary metabolites, including
“Cannabis sativa L. is one of the most-studied species for its phytochemistry due to the abundance of secondary metabolites, including 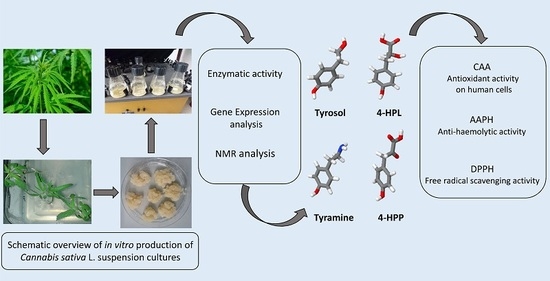
 “Hypercholesterolemia (HC) is a major risk factor for cardiovascular (CV) diseases, that are the major cause of mortality worldwide.
“Hypercholesterolemia (HC) is a major risk factor for cardiovascular (CV) diseases, that are the major cause of mortality worldwide.
 “We used mouse microglial cells in culture activated by lipopolysaccharide (LPS, 10 ng/ml) to study the anti-inflammatory potential of
“We used mouse microglial cells in culture activated by lipopolysaccharide (LPS, 10 ng/ml) to study the anti-inflammatory potential of  “The purpose of this study was to investigate structure of
“The purpose of this study was to investigate structure of 
 “The healthy benefits of hemp (
“The healthy benefits of hemp ( “Hemp (
“Hemp (