 “Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol.
“Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol.
Cannabinoids elicit a wide range of central and peripheral effects mostly mediated through cannabinoid receptors. There are two types of specific Gi/o-protein-coupled receptors cloned so far, called CB1 and CB2, although an existence of additional cannabinoid-binding receptors has been suggested. CB1 and CB2 differ in their predicted amino acid sequence, tissue distribution, physiological role and signaling mechanisms.
Significant alterations of a balance in the cannabinoid system between the levels of endogenous ligands and their receptors occur during malignant transformation in various types of cancer, including gliomas.
Cannabinoids exert anti-proliferative action in tumor cells.
Induction of cell death by cannabinoid treatment relies on the generation of a pro-apoptotic sphingolipid ceramide and disruption of signaling pathways crucial for regulation of cellular proliferation, differentiation or apoptosis. Increased ceramide levels lead also to ER-stress and autophagy in drug-treated glioblastoma cells.
Beyond blocking of tumor cells proliferation cannabinoids inhibit invasiveness, angiogenesis and the stem cell-like properties of glioma cells, showing profound activity in the complex tumor microenvironment. Advances in translational research on cannabinoid signaling led to clinical investigations on the use of cannabinoids in treatments of glioblastomas.”
https://www.ncbi.nlm.nih.gov/pubmed/32034716
https://link.springer.com/chapter/10.1007%2F978-3-030-30651-9_11
“Cannabinoids exert anti-proliferative action in tumor cells.” https://www.ncbi.nlm.nih.gov/pubmed/22879071
“A glioma is a primary brain tumor that originates from the supportive cells of the brain, called glial cells.” http://neurosurgery.ucla.edu/body.cfm?id=159
“Remarkably, cannabinoids kill glioma cells selectively and can protect non-transformed glial cells from death.” http://www.ncbi.nlm.nih.gov/pubmed/15275820

 “In the last decade, we have observed an increased public and scientific interest in the clinical applications of medical cannabis.
“In the last decade, we have observed an increased public and scientific interest in the clinical applications of medical cannabis. “Endo-, phyto- and synthetic cannabinoids have been proposed as promising anti-cancer agents able to impair cancer cells’ behavior without affecting their non-transformed counterparts.
“Endo-, phyto- and synthetic cannabinoids have been proposed as promising anti-cancer agents able to impair cancer cells’ behavior without affecting their non-transformed counterparts.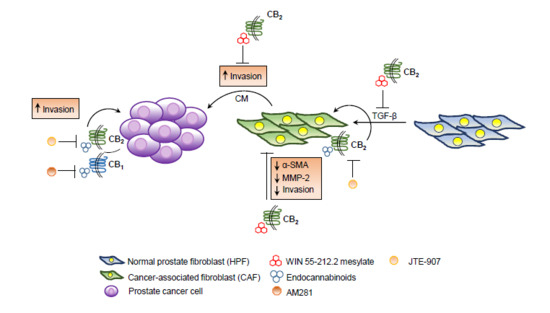
 “The aim of this review article is to summarize current knowledge about the role of cannabinoids and cannabinoid receptors in tumor disease modulation and to evaluate comprehensively the use of cannabinoids in cancer patients.
“The aim of this review article is to summarize current knowledge about the role of cannabinoids and cannabinoid receptors in tumor disease modulation and to evaluate comprehensively the use of cannabinoids in cancer patients. “Several natural compounds have demonstrated potential for the treatment of central nervous system disorders such as ischemic cerebrovascular disease, glioblastoma, neuropathic pain, neurodegenerative diseases, multiple sclerosis and migraine.
“Several natural compounds have demonstrated potential for the treatment of central nervous system disorders such as ischemic cerebrovascular disease, glioblastoma, neuropathic pain, neurodegenerative diseases, multiple sclerosis and migraine. “Cannabis was used for cancer patients as early as about 2500 years ago.
“Cannabis was used for cancer patients as early as about 2500 years ago.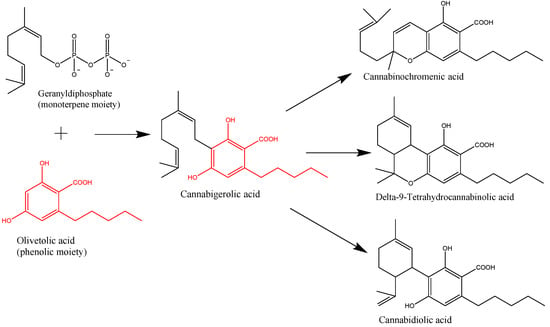
 “WIN55,212-2 (WIN) is a synthetic agonist of
“WIN55,212-2 (WIN) is a synthetic agonist of 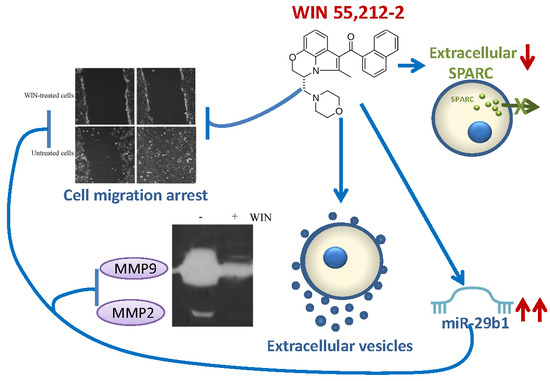
 “Cannabinoids exhibit anti-inflammatory and antitumorigenic properties.
“Cannabinoids exhibit anti-inflammatory and antitumorigenic properties.