 “In the absence of effective antivirals and vaccination, the pandemic of COVID-19 remains the most significant challenge to our health care system in decades. There is an urgent need for definitive therapeutic intervention.
“In the absence of effective antivirals and vaccination, the pandemic of COVID-19 remains the most significant challenge to our health care system in decades. There is an urgent need for definitive therapeutic intervention.
Clinical reports indicate that the cytokine storm associated with acute respiratory distress syndrome (ARDS) is the leading cause of mortality in severe cases of some respiratory viral infections, including COVID-19.
In recent years, cannabinoids have been investigated extensively due to their potential effects on the human body. Among all cannabinoids, cannabidiol (CBD) has demonstrated potent anti-inflammatory effects in a variety of pathological conditions. Therefore, it is logical to explore whether CBD can reduce the cytokine storm and treat ARDS.
Materials and Methods: In this study, we show that intranasal application of Poly(I:C), a synthetic analogue of viral double-stranded RNA, simulated symptoms of severe viral infections inducing signs of ARDS and cytokine storm.
Discussion: The administration of CBD downregulated the level of proinflammatory cytokines and ameliorated the clinical symptoms of Poly I:C-induced ARDS.
Conclusion: Our results suggest a potential protective role for CBD during ARDS that may extend CBD as part of the treatment of COVID-19 by reducing the cytokine storm, protecting pulmonary tissues, and re-establishing inflammatory homeostasis.”

 “Cannabidiol (CBD) is a non-intoxicating and generally well-tolerated constituent of cannabis which exhibits potential beneficial properties in a wide range of diseases, including cardiovascular disorders.
“Cannabidiol (CBD) is a non-intoxicating and generally well-tolerated constituent of cannabis which exhibits potential beneficial properties in a wide range of diseases, including cardiovascular disorders.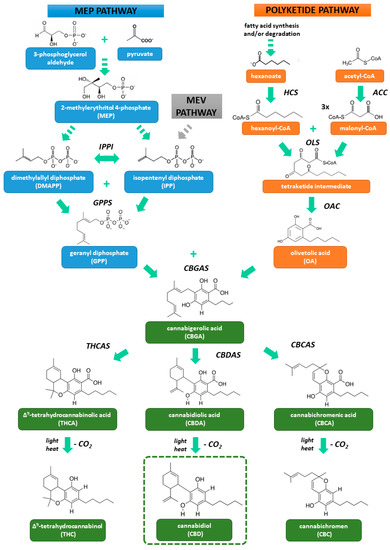
 “Emotional dysregulation and anxiety are common in people at clinical high risk for psychosis (CHR) and are associated with altered neural responses to emotional stimuli in the striatum and medial temporal lobe.
“Emotional dysregulation and anxiety are common in people at clinical high risk for psychosis (CHR) and are associated with altered neural responses to emotional stimuli in the striatum and medial temporal lobe.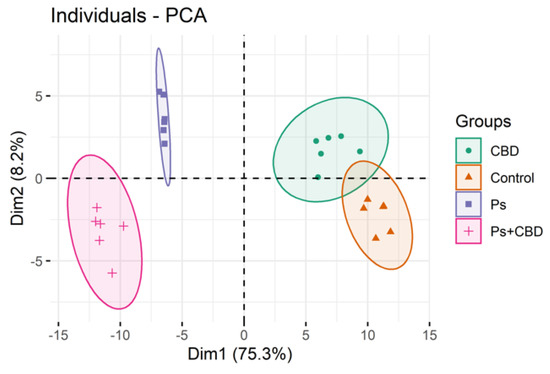
 “Highly purified cannabidiol (CBD) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut syndrome or Dravet syndrome in randomized, double-blind, add-on, controlled phase 3 trials.
“Highly purified cannabidiol (CBD) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut syndrome or Dravet syndrome in randomized, double-blind, add-on, controlled phase 3 trials. “Mutations in SYNGAP1 are associated with developmental delay, epilepsy, and autism spectrum disorder (ASD). Epilepsy is often drug-resistant in this syndrome with frequent drop attacks.
“Mutations in SYNGAP1 are associated with developmental delay, epilepsy, and autism spectrum disorder (ASD). Epilepsy is often drug-resistant in this syndrome with frequent drop attacks. “Hemp (Cannabis sativa L.) seed contains high contents of various nutrients, including fatty acids and proteins.
“Hemp (Cannabis sativa L.) seed contains high contents of various nutrients, including fatty acids and proteins.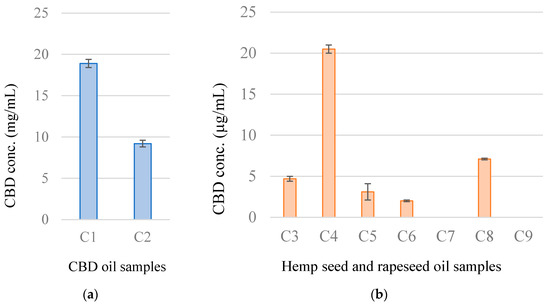
 “The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator.
“The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator. “Autism spectrum disorder (ASD) is a multifactorial, pervasive neurodevelopmental disorder defined by the core symptoms of significant impairment in social interaction and communication as well as restricted, repetitive patterns of behavior. In addition to these core behaviors, persons with ASD frequently have associated noncore behavioral disturbance (ie, self-injury, aggression), as well as several medical comorbidities. Currently, no effective treatment exists for the core symptoms of ASD.
“Autism spectrum disorder (ASD) is a multifactorial, pervasive neurodevelopmental disorder defined by the core symptoms of significant impairment in social interaction and communication as well as restricted, repetitive patterns of behavior. In addition to these core behaviors, persons with ASD frequently have associated noncore behavioral disturbance (ie, self-injury, aggression), as well as several medical comorbidities. Currently, no effective treatment exists for the core symptoms of ASD.