 “Four pivotal randomized placebo-controlled trials have demonstrated that adjunctive therapy with cannabidiol (CBD) improves seizure control in patients with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS).
“Four pivotal randomized placebo-controlled trials have demonstrated that adjunctive therapy with cannabidiol (CBD) improves seizure control in patients with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS).
Between 47% and 68% of patients allocated to CBD treatment in these trials were receiving clobazam (CLB), which shows complex interactions with CBD resulting, in particular, in a 3.4- to 5-fold increase in plasma concentration of the active metabolite norclobazam. This raises concern as to the role played by these interactions in determining the reduction in seizure frequency in CBD-treated patients, and the question of whether CBD per se has clinically evident antiseizure effects.
We appraised available evidence on the clinical consequences of the CBD-CLB interaction, focusing on subgroup analyses of seizure outcomes in patients on and off CLB comedication in the pivotal CBD trials, as provided by the European Medicines Agency Public Assessment Report.
Evaluation of the results of individual trials clearly showed that improvement in seizure control over placebo was greater when CBD was added on to CLB than when it was added on to other medications. However, seizure control was also improved in patients off CLB, and despite the small sample size the difference vs placebo was statistically significant for the 10 mg/kg/d dose in one of the two LGS trials.
Stronger evidence for an antiseizure effect of CBD independent of an interaction with CLB emerges from meta-analyses of seizure outcomes in the pooled population of LGS and DS patients not receiving CLB comedication.
Although these results need to be interpreted taking into account methodological limitations, they provide the best clinical evidence to date that CBD exerts therapeutic effects in patients with epilepsy that are independent of its interaction with CLB. Greater antiseizure effects, and a greater burden of adverse effects, are observed when CBD is combined with CLB.”

 “The market for products featuring hemp extracts is large and growing larger. However, safety concerns have been raised by medical and regulatory agencies. Post marketing surveillance of full spectrum hemp extract (FSHE) products manufactured and distributed by CV Sciences (CVSI) and traded under the brand PlusCBD™ was conducted over a 2-year period (2018-2019). The safety of these products was assessed by analyzing adverse events reports.
“The market for products featuring hemp extracts is large and growing larger. However, safety concerns have been raised by medical and regulatory agencies. Post marketing surveillance of full spectrum hemp extract (FSHE) products manufactured and distributed by CV Sciences (CVSI) and traded under the brand PlusCBD™ was conducted over a 2-year period (2018-2019). The safety of these products was assessed by analyzing adverse events reports. “Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.
“Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain. “Cannabidiol (CBD) and cannabigerol (CBG) are Cannabis sativa terpenophenols.
“Cannabidiol (CBD) and cannabigerol (CBG) are Cannabis sativa terpenophenols.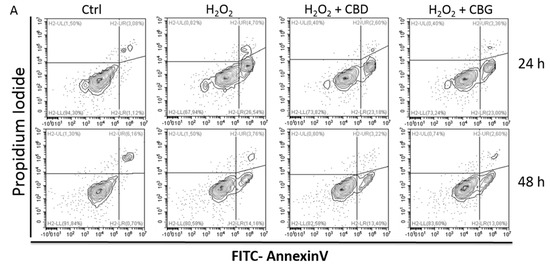
 “In this study cannabidiol (CBD) was administered orally to determine its effects and mechanisms in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). We hypothesized that 75 mg/kg of oral CBD given for 5 days after initiation of disease would reduce EAE severity through suppression of either the early peripheral immune or late neuroimmune response.
“In this study cannabidiol (CBD) was administered orally to determine its effects and mechanisms in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). We hypothesized that 75 mg/kg of oral CBD given for 5 days after initiation of disease would reduce EAE severity through suppression of either the early peripheral immune or late neuroimmune response.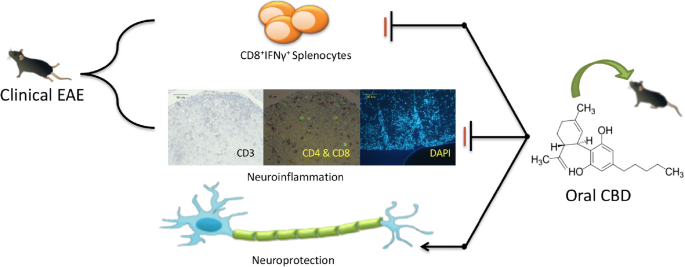
 “Cannabidiol (CBD), the major non-psychoactive constituent of Cannabis sativa L., has gained traction as a potential treatment for intractable chronic pain in many conditions. Clinical evidence suggests that CBD provides therapeutic benefit in certain forms of epilepsy and imparts analgesia in certain conditions, and improves quality of life.
“Cannabidiol (CBD), the major non-psychoactive constituent of Cannabis sativa L., has gained traction as a potential treatment for intractable chronic pain in many conditions. Clinical evidence suggests that CBD provides therapeutic benefit in certain forms of epilepsy and imparts analgesia in certain conditions, and improves quality of life.
 “Few models exist that can control for placebo and expectancy effects commonly observed in clinical trials measuring ‘
“Few models exist that can control for placebo and expectancy effects commonly observed in clinical trials measuring ‘ “The cannabis plant has been widely researched for many therapeutic indications and found to be effective in many chronic conditions such as epilepsy, neuropathic or chronic pain and more. However, biased opinion against compounds of the plant, regulatory as well as compounding challenges have led to very few approved medicinal products. Those formulations which are approved are dosed several times a day, creating an unmet need for controlled release (CR) formulations of
“The cannabis plant has been widely researched for many therapeutic indications and found to be effective in many chronic conditions such as epilepsy, neuropathic or chronic pain and more. However, biased opinion against compounds of the plant, regulatory as well as compounding challenges have led to very few approved medicinal products. Those formulations which are approved are dosed several times a day, creating an unmet need for controlled release (CR) formulations of  “Two patient case reports are presented describing the use of cannabidiol (CBD) for the symptomatic relief of a lumbar compression fracture and in the mitigation of thoracic discomfort and dysesthesia secondary to a surgically resected meningioma.
“Two patient case reports are presented describing the use of cannabidiol (CBD) for the symptomatic relief of a lumbar compression fracture and in the mitigation of thoracic discomfort and dysesthesia secondary to a surgically resected meningioma.