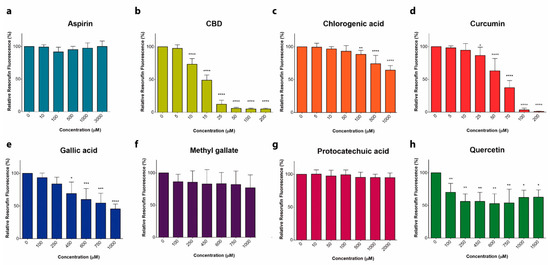
 “Anticancer activity of different phenols is documented, but underlying mechanisms remain elusive. Recently, we have shown that cannabidiol kills the cells of acute lymphoblastic leukemia (ALL) by a direct interaction with mitochondria, with their consequent dysfunction.
“Anticancer activity of different phenols is documented, but underlying mechanisms remain elusive. Recently, we have shown that cannabidiol kills the cells of acute lymphoblastic leukemia (ALL) by a direct interaction with mitochondria, with their consequent dysfunction.
In the present study, cytotoxic effects of several phenolic compounds against human the T-ALL cell line Jurkat were tested by means of resazurin-based metabolic assay. To unravel underlying mechanisms, mitochondrial membrane potential (∆Ψm) and [Ca2+]m measurements were undertaken, and reactive oxygen species generation and cell death were evaluated by flow cytometry.
Three out of eight tested phenolics, cannabidiol, curcumin and quercetin, which displayed a significant cytotoxic effect, also dissipated the ∆Ψm and induced a significant [Ca2+]m increase, whereas inefficient phenols did not.
Dissipation of the ∆Ψm by cannabidiol was prevented by cyclosporine A and reverted by Ru360, inhibitors of the permeation transition pore and mitochondrial Ca2+ uniporter, respectively. Ru360 prevented the phenol-induced [Ca2+]m rise, but neither cyclosporine A nor Ru360 affected the curcumin- and quercetin-induced ∆Ψm depolarization. Ru360 impeded the curcumin- and cannabidiol-induced cell death.
Thus, all three phenols exert their antileukemic activity via mitochondrial Ca2+ overload, whereas curcumin and quercetin suppress the metabolism of leukemic cells by direct mitochondrial uncoupling.”
https://pubmed.ncbi.nlm.nih.gov/33379175/
https://www.mdpi.com/1422-0067/22/1/204

 “Stem cell therapy promotes tissue regeneration and wound healing. Efforts have been made to prime stem cells to enhance their regenerative abilities.
“Stem cell therapy promotes tissue regeneration and wound healing. Efforts have been made to prime stem cells to enhance their regenerative abilities. “Effective treatment choices to the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) are limited because of the absence of effective target-based therapeutics. The main object of the current research was to estimate the antiviral activity of cannabinoids (CBDs) against the human coronavirus SARS-CoV-2.
“Effective treatment choices to the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) are limited because of the absence of effective target-based therapeutics. The main object of the current research was to estimate the antiviral activity of cannabinoids (CBDs) against the human coronavirus SARS-CoV-2.
 “Cannabis sativa is a well-known plant which has been of benefit since ancient times in several medicinal systems, including Chinese, Indian, Greek and Egyptian ones.
“Cannabis sativa is a well-known plant which has been of benefit since ancient times in several medicinal systems, including Chinese, Indian, Greek and Egyptian ones. “UV radiation is a well-established environmental risk factor known to cause oxidative stress and disrupt the metabolism of keratinocyte phospholipids. Cannabidiol (CBD) is a phytocannabinoid with anti-inflammatory and antioxidant effects.
“UV radiation is a well-established environmental risk factor known to cause oxidative stress and disrupt the metabolism of keratinocyte phospholipids. Cannabidiol (CBD) is a phytocannabinoid with anti-inflammatory and antioxidant effects.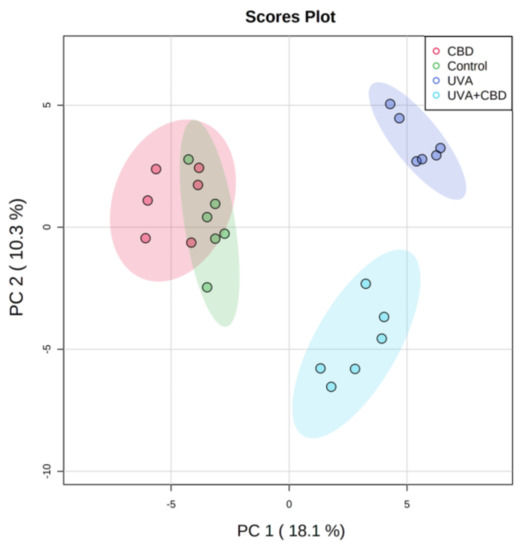
 “Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy.
“Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy. “Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
“Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant. “Cannabidiol (CBD) has anti-tumorigenic activity. However, the anti-cancer effect of CBD on head and neck squamous cell carcinoma (HNSCC) remains unclear. The cytotoxicity of CBD on HNSCC was analyzed using cell survival and colony-forming assays in vitro.
“Cannabidiol (CBD) has anti-tumorigenic activity. However, the anti-cancer effect of CBD on head and neck squamous cell carcinoma (HNSCC) remains unclear. The cytotoxicity of CBD on HNSCC was analyzed using cell survival and colony-forming assays in vitro. “The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.
“The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.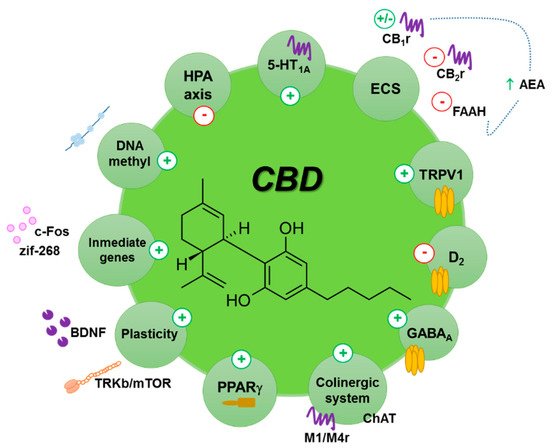
 “With the current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality.
“With the current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality.