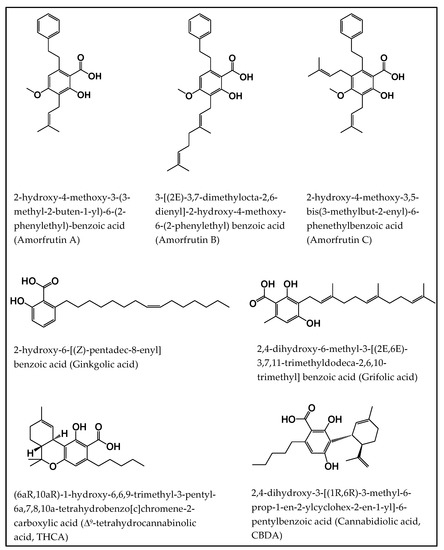
 “Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses. Increasing reports suggest that this phenolic compound and its derivatives, collectively termed salicylates, not only regulate plant defense but also have beneficial effects on human health. Both natural and synthetic salicylates are known to have multiple targets in humans, thereby exhibiting various appreciating pharmacological roles, including anti-inflammatory, anticancer, neuroprotective, antidiabetic effects, and so on. The role of some salicylates, such as acetylsalicylic acid (aspirin), 5-aminosalicylic acid (mesalazine), and amorfrutins in human diseases has been well studied in vitro. However, their clinical significance in different diseases is largely unknown. Based on recent studies, five natural salicylates, including amorfrutin, ginkgolic acid, grifolic acid, tetrahydrocannabinolic acid, and cannabidiolic acid, showed potential roles in different challenging human diseases. This review summarizes together some of the recent information on multitarget regulatory activities of these natural salicylates and their pharmacological roles in human health.”
“Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses. Increasing reports suggest that this phenolic compound and its derivatives, collectively termed salicylates, not only regulate plant defense but also have beneficial effects on human health. Both natural and synthetic salicylates are known to have multiple targets in humans, thereby exhibiting various appreciating pharmacological roles, including anti-inflammatory, anticancer, neuroprotective, antidiabetic effects, and so on. The role of some salicylates, such as acetylsalicylic acid (aspirin), 5-aminosalicylic acid (mesalazine), and amorfrutins in human diseases has been well studied in vitro. However, their clinical significance in different diseases is largely unknown. Based on recent studies, five natural salicylates, including amorfrutin, ginkgolic acid, grifolic acid, tetrahydrocannabinolic acid, and cannabidiolic acid, showed potential roles in different challenging human diseases. This review summarizes together some of the recent information on multitarget regulatory activities of these natural salicylates and their pharmacological roles in human health.”
https://pubmed.ncbi.nlm.nih.gov/33260759/
https://www.mdpi.com/1422-0067/21/23/9049

 “Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy.
“Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy. “Rett syndrome (RTT) is a rare neurologic disorder, characterized by severe behavioural and physiological symptoms. RTT is caused by mutations in the MECP2 gene in about 95% of cases and to date no cure is available.
“Rett syndrome (RTT) is a rare neurologic disorder, characterized by severe behavioural and physiological symptoms. RTT is caused by mutations in the MECP2 gene in about 95% of cases and to date no cure is available. “The emergence of multi-drug resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) causes a major threat to public health due to its limited therapeutic options.
“The emergence of multi-drug resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) causes a major threat to public health due to its limited therapeutic options.
 “Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
“Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
 “Rationale: When acutely administered intraperitoneally, the non-psychoactive cannabinoid cannabidiol (CBD), its acidic precursor cannabidiolic acid (CBDA) and a stable methyl ester of CBDA (HU-580) reduce lithium chloride (LiCl)-induced conditioned gaping in male rats (a selective preclinical model of acute nausea) via activation of the serotonin 1A (5-HT1A) receptor.
“Rationale: When acutely administered intraperitoneally, the non-psychoactive cannabinoid cannabidiol (CBD), its acidic precursor cannabidiolic acid (CBDA) and a stable methyl ester of CBDA (HU-580) reduce lithium chloride (LiCl)-induced conditioned gaping in male rats (a selective preclinical model of acute nausea) via activation of the serotonin 1A (5-HT1A) receptor. “The major phytocannabinoid cannabidiol (
“The major phytocannabinoid cannabidiol ( “Cannabidiolic acid methyl ester (HU-580) is a more stable compound than cannabidiolic acid (CBDA) which has been shown to be effective in reducing nausea, anxiety, depression behaviors in animal models.
“Cannabidiolic acid methyl ester (HU-580) is a more stable compound than cannabidiolic acid (CBDA) which has been shown to be effective in reducing nausea, anxiety, depression behaviors in animal models.
