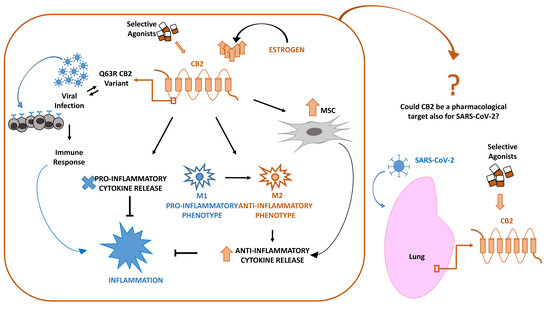
 “In late December 2019, a novel coronavirus (SARS-CoV-2 or CoV-19) appeared in Wuhan, China, causing a global pandemic. SARS-CoV-2 causes mild to severe respiratory tract inflammation, often developing into lung fibrosis with thrombosis in pulmonary small vessels and causing even death. COronaVIrus Disease (COVID-19) patients manifest exacerbated inflammatory and immune responses, cytokine storm, prevalence of pro-inflammatory M1 macrophages and increased levels of resident and circulating immune cells. Men show higher susceptibility to SARS-CoV-2 infection than women, likely due to estrogens production. The protective role of estrogens, as well as an immune-suppressive activity that limits the excessive inflammation, can be mediated by cannabinoid receptor type 2 (CB2). The role of this receptor in modulating inflammation and immune response is well documented in fact in several settings. The stimulation of CB2 receptors is known to limit the release of pro-inflammatory cytokines, shift the macrophage phenotype towards the anti-inflammatory M2 type and enhance the immune-modulating properties of mesenchymal stromal cells. For these reasons, we hypothesize that CB2 receptor can be a therapeutic target in COVID-19 pandemic emergency.”
“In late December 2019, a novel coronavirus (SARS-CoV-2 or CoV-19) appeared in Wuhan, China, causing a global pandemic. SARS-CoV-2 causes mild to severe respiratory tract inflammation, often developing into lung fibrosis with thrombosis in pulmonary small vessels and causing even death. COronaVIrus Disease (COVID-19) patients manifest exacerbated inflammatory and immune responses, cytokine storm, prevalence of pro-inflammatory M1 macrophages and increased levels of resident and circulating immune cells. Men show higher susceptibility to SARS-CoV-2 infection than women, likely due to estrogens production. The protective role of estrogens, as well as an immune-suppressive activity that limits the excessive inflammation, can be mediated by cannabinoid receptor type 2 (CB2). The role of this receptor in modulating inflammation and immune response is well documented in fact in several settings. The stimulation of CB2 receptors is known to limit the release of pro-inflammatory cytokines, shift the macrophage phenotype towards the anti-inflammatory M2 type and enhance the immune-modulating properties of mesenchymal stromal cells. For these reasons, we hypothesize that CB2 receptor can be a therapeutic target in COVID-19 pandemic emergency.”
https://pubmed.ncbi.nlm.nih.gov/32471272/
https://www.mdpi.com/1422-0067/21/11/3809

 “Historical relevance: Cannabis sativa L. (C. sativa) is a plant whose use as a therapeutic agent shares its origins with the first Far East’s human societies. Cannabis has been used not only for recreational purposes, but as a food to obtain textile fibers, to produce hemp paper, to treat many physical and mental disorders.
“Historical relevance: Cannabis sativa L. (C. sativa) is a plant whose use as a therapeutic agent shares its origins with the first Far East’s human societies. Cannabis has been used not only for recreational purposes, but as a food to obtain textile fibers, to produce hemp paper, to treat many physical and mental disorders. “While natural Δ9-tetrahidrocannabinol (Δ9THC),
“While natural Δ9-tetrahidrocannabinol (Δ9THC),  “HIV infection affects an estimated 38 million people. Approximately 50% of HIV patients exhibit neurocognitive dysfunction termed HIV-Associated Neurocognitive Disorder (HAND). HAND is a consequence of chronic low-level neuroinflammation due to HIV entry into the brain. Initially, monocytes become activated in circulation and traffic to the brain. Monocytes, when activated, become susceptible to infection by HIV and can then carry the virus across the blood brain barrier. Once in the brain, activated monocytes secrete chemokines, which recruit virus-specific CD8+ T cells into the brain to further promote neuroinflammation. HAND is closely linked to systemic inflammation driven, in part, by HIV but is also due to persistent translocation of microorganisms across the GI tract. Persistent anti-viral responses in the GI tract compromise microbial barrier integrity. Indeed, HIV patients can exhibit remarkably high levels of activated (CD16+) monocytes in circulation.
“HIV infection affects an estimated 38 million people. Approximately 50% of HIV patients exhibit neurocognitive dysfunction termed HIV-Associated Neurocognitive Disorder (HAND). HAND is a consequence of chronic low-level neuroinflammation due to HIV entry into the brain. Initially, monocytes become activated in circulation and traffic to the brain. Monocytes, when activated, become susceptible to infection by HIV and can then carry the virus across the blood brain barrier. Once in the brain, activated monocytes secrete chemokines, which recruit virus-specific CD8+ T cells into the brain to further promote neuroinflammation. HAND is closely linked to systemic inflammation driven, in part, by HIV but is also due to persistent translocation of microorganisms across the GI tract. Persistent anti-viral responses in the GI tract compromise microbial barrier integrity. Indeed, HIV patients can exhibit remarkably high levels of activated (CD16+) monocytes in circulation.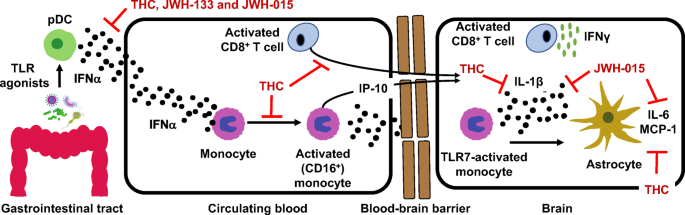
 “The antioxidant and CB2 receptor agonist properties of Δ9-tetrahydrocannabivarin (Δ9-THCV) afforded neuroprotection in experimental Parkinson’s disease (PD), whereas its CB1 receptor antagonist profile at doses lower than 5 mg/kg caused anti-hypokinetic effects.
“The antioxidant and CB2 receptor agonist properties of Δ9-tetrahydrocannabivarin (Δ9-THCV) afforded neuroprotection in experimental Parkinson’s disease (PD), whereas its CB1 receptor antagonist profile at doses lower than 5 mg/kg caused anti-hypokinetic effects. “Lenabasum is an oral synthetic
“Lenabasum is an oral synthetic  “Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and
“Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and 
 “Borderline Personality Disorder (BPD) is a chronic debilitating psychiatric disorder characterized mainly by emotional instability, chaotic interpersonal relationships, cognitive disturbance (e.g. dissociation and suicidal thoughts) and maladaptive behaviors. BPD has a high rate of comorbidity with other mental disorders and high burden on society.
“Borderline Personality Disorder (BPD) is a chronic debilitating psychiatric disorder characterized mainly by emotional instability, chaotic interpersonal relationships, cognitive disturbance (e.g. dissociation and suicidal thoughts) and maladaptive behaviors. BPD has a high rate of comorbidity with other mental disorders and high burden on society.