 “The global cancer burden is significantly increasing at an alarming rate affecting patients, relatives, communities, and health-care system. Cancer patients require adequate pain relief and palliative care throughout the life course, especially in terminal illness. Although opioid treatment is successful in majority of patients, around 40% do not achieve enough analgesia or are prone to serious side effects and toxicity. The treatment of medical conditions with cannabis and cannabinoid compounds is constantly expanding. This review organizes the current knowledge in the context of SNPs associated with opioids and nonopioids and its clinical consequences in pain management and pharmacogenetic targets of cannabinoids, for use in clinical practice.”
“The global cancer burden is significantly increasing at an alarming rate affecting patients, relatives, communities, and health-care system. Cancer patients require adequate pain relief and palliative care throughout the life course, especially in terminal illness. Although opioid treatment is successful in majority of patients, around 40% do not achieve enough analgesia or are prone to serious side effects and toxicity. The treatment of medical conditions with cannabis and cannabinoid compounds is constantly expanding. This review organizes the current knowledge in the context of SNPs associated with opioids and nonopioids and its clinical consequences in pain management and pharmacogenetic targets of cannabinoids, for use in clinical practice.”
Tag Archives: cannabinoid receptors
Role of cannabis in inflammatory bowel diseases.
 “For many centuries, cannabis (marijuana) has been used for both recreational and medicinal purposes. Currently, there are about 192 million cannabis users worldwide, constituting approximately 3.9% of the global population. Cannabis comprises more than 70 aromatic hydrocarbon compounds known as cannabinoids. Endogenous circulating cannabinoids, or endocannabinoids, such as anandamide and 2-arachidonoyl-glycerol, their metabolizing enzymes (fatty acid amide hydrolase and monoacylglycerol lipase) and 2 G-protein coupled cannabinoid receptors, CB1 and CB2, together represent the endocannabinoid system and are present throughout the human body. In the gastrointestinal (GI) tract, the activated endocannabinoid system reduces gut motility, intestinal secretion and epithelial permeability, and induces inflammatory leukocyte recruitment and immune modulation through the cannabinoid receptors present in the enteric nervous and immune systems. Because of the effects of cannabinoids on the GI tract, attempts have been made to investigate their medicinal properties, particularly for GI disorders such as pancreatitis, hepatitis, and inflammatory bowel diseases (IBD). The effects of cannabis on IBD have been elucidated in several small observational and placebo-controlled studies, but with varied results. The small sample size and short follow-up duration in these studies make it difficult to show the clear benefits of cannabis in IBD. However, cannabis is now being considered as a potential drug for inflammatory GI conditions, particularly IBD, because of its spreading legalization in the United States and other countries and the growing trend in its use. More high-quality controlled studies are warranted to elucidate the mechanism and benefits of cannabis use as a possible option in IBD management.”
“For many centuries, cannabis (marijuana) has been used for both recreational and medicinal purposes. Currently, there are about 192 million cannabis users worldwide, constituting approximately 3.9% of the global population. Cannabis comprises more than 70 aromatic hydrocarbon compounds known as cannabinoids. Endogenous circulating cannabinoids, or endocannabinoids, such as anandamide and 2-arachidonoyl-glycerol, their metabolizing enzymes (fatty acid amide hydrolase and monoacylglycerol lipase) and 2 G-protein coupled cannabinoid receptors, CB1 and CB2, together represent the endocannabinoid system and are present throughout the human body. In the gastrointestinal (GI) tract, the activated endocannabinoid system reduces gut motility, intestinal secretion and epithelial permeability, and induces inflammatory leukocyte recruitment and immune modulation through the cannabinoid receptors present in the enteric nervous and immune systems. Because of the effects of cannabinoids on the GI tract, attempts have been made to investigate their medicinal properties, particularly for GI disorders such as pancreatitis, hepatitis, and inflammatory bowel diseases (IBD). The effects of cannabis on IBD have been elucidated in several small observational and placebo-controlled studies, but with varied results. The small sample size and short follow-up duration in these studies make it difficult to show the clear benefits of cannabis in IBD. However, cannabis is now being considered as a potential drug for inflammatory GI conditions, particularly IBD, because of its spreading legalization in the United States and other countries and the growing trend in its use. More high-quality controlled studies are warranted to elucidate the mechanism and benefits of cannabis use as a possible option in IBD management.”
https://www.ncbi.nlm.nih.gov/pubmed/32127734
http://www.annalsgastro.gr/files/journals/1/earlyview/2020/ev-02-2020-03-AG4866-0452.pdf
Endocannabinoid Modulation of Microglial Phenotypes in Neuropathology.
 “Microglia, the resident immune cells of the central nervous system, mediate brain homeostasis by controlling neuronal proliferation/differentiation and synaptic activity. In response to external signals from neuropathological conditions, homeostatic (M0) microglia can adopt one of two activation states: the classical (M1) activation state, which secretes mediators of the proinflammatory response, and the alternative (M2) activation state, which presumably mediates the resolution of neuroinflammation and tissue repair/remodeling.
“Microglia, the resident immune cells of the central nervous system, mediate brain homeostasis by controlling neuronal proliferation/differentiation and synaptic activity. In response to external signals from neuropathological conditions, homeostatic (M0) microglia can adopt one of two activation states: the classical (M1) activation state, which secretes mediators of the proinflammatory response, and the alternative (M2) activation state, which presumably mediates the resolution of neuroinflammation and tissue repair/remodeling.
Since chronic inflammatory activation of microglia is correlated with several neurodegenerative diseases, functional modulation of microglial phenotypes has been considered as a potential therapeutic strategy.
The endocannabinoid (eCB) system, composed of cannabinoid receptors and ligands and their metabolic/biosynthetic enzymes, has been shown to activate anti-inflammatory signaling pathways that modulate immune cell functions. Growing evidence has demonstrated that endogenous, synthetic, and plant-derived eCB agonists possess therapeutic effects on several neuropathologies; however, the molecular mechanisms that mediate the anti-inflammatory effects have not yet been identified.
Over the last decade, it has been revealed that the eCB system modulates microglial activation and population. In this review, we thoroughly examine recent studies on microglial phenotype modulation by eCB in neuroinflammatory and neurodegenerative disease conditions.
We hypothesize that cannabinoid 2 receptor (CB2R) signaling shifts the balance of expression between neuroinflammatory (M1-type) genes, neuroprotective (M2-type) genes, and homeostatic (M0-type) genes toward the latter two gene expressions, by which microglia acquire therapeutic functionality.”
https://www.ncbi.nlm.nih.gov/pubmed/32117037
https://www.frontiersin.org/articles/10.3389/fneur.2020.00087/full
Cannabinoids and Hormone Receptor-Positive Breast Cancer Treatment.
 “Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).
“Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).
Endogenous cannabinoids together with cannabinoid receptor 1 and 2 (CB1, CB2) constitute the basis of the endocannabinoid system.
Interactions of cannabinoids with hypothalamic-pituitary-gonadal axis hormones are well documented, and two studies found a positive correlation between peak plasma endogenous cannabinoid anandamide with peak plasma 17β-estradiol, luteinizing hormone and follicle-stimulating hormone levels at ovulation in healthy premenopausal women. Do cannabinoids have an effect on HR+ BC? In this paper we review known and possible interactions between cannabinoids and specific HR+ BC treatments.
In preclinical studies, CB1 and CB2 agonists (i.e., anandamide, THC) have been shown to inhibit the proliferation of ER positive BC cell lines.
There is less evidence for antitumor cannabinoid action in HR+ BC in animal models and there are no clinical trials exploring the effects of cannabinoids on HR+ BC treatment outcomes. Two studies have shown that tamoxifen and several other selective estrogen receptor modulators (SERM) can act as inverse agonists on CB1 and CB2, an interaction with possible clinical consequences. In addition, cannabinoid action could interact with other commonly used endocrine and targeted therapies used in the treatment of HR+ BC.”
https://www.ncbi.nlm.nih.gov/pubmed/32106399
https://www.mdpi.com/2072-6694/12/3/525
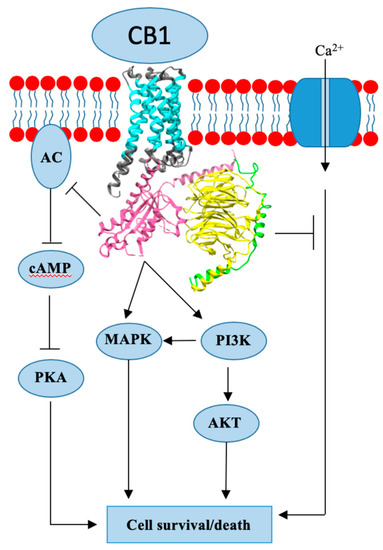
Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease.
 “Herein, 11 general types of natural cannabinoids from Cannabis sativa as well as 50 (-)-CBD analogues with therapeutic potential were described. The underlying molecular mechanisms of CBD as a therapeutic candidate for epilepsy and neurodegenerative diseases were comprehensively clarified. CBD indirectly acts as an endogenous cannabinoid receptor agonist to exert its neuroprotective effects. CBD also promotes neuroprotection through different signal transduction pathways mediated indirectly by cannabinoid receptors. Furthermore, CBD prevents the glycogen synthase kinase 3β (GSK-3β) hyperphosphorylation caused by Aβ and may be developed as a new therapeutic candidate for Alzheimer’s disease.”
“Herein, 11 general types of natural cannabinoids from Cannabis sativa as well as 50 (-)-CBD analogues with therapeutic potential were described. The underlying molecular mechanisms of CBD as a therapeutic candidate for epilepsy and neurodegenerative diseases were comprehensively clarified. CBD indirectly acts as an endogenous cannabinoid receptor agonist to exert its neuroprotective effects. CBD also promotes neuroprotection through different signal transduction pathways mediated indirectly by cannabinoid receptors. Furthermore, CBD prevents the glycogen synthase kinase 3β (GSK-3β) hyperphosphorylation caused by Aβ and may be developed as a new therapeutic candidate for Alzheimer’s disease.”
https://www.ncbi.nlm.nih.gov/pubmed/32109623
“For AD treatment, CBD can rescue the production of neurofibrillary tangles and inhibit neuronal apoptosis.”
https://www.sciencedirect.com/science/article/abs/pii/S0223523420301306?via%3Dihub
A Review of Scientific Evidence for THC:CBD Oromucosal Spray (Nabiximols) in the Management of Chronic Pain.
 “The 20% prevalence of chronic pain in the general population is a major health concern given the often profound associated impairment of daily activities, employment status, and health-related quality of life in sufferers. Resource utilization associated with chronic pain represents an enormous burden for healthcare systems. Although analgesia based on the World Health Organization’s pain ladder continues to be the mainstay of chronic pain management, aside from chronic cancer pain or end-of-life care, prolonged use of non-steroidal anti-inflammatory drugs or opioids to manage chronic pain is rarely sustainable.
“The 20% prevalence of chronic pain in the general population is a major health concern given the often profound associated impairment of daily activities, employment status, and health-related quality of life in sufferers. Resource utilization associated with chronic pain represents an enormous burden for healthcare systems. Although analgesia based on the World Health Organization’s pain ladder continues to be the mainstay of chronic pain management, aside from chronic cancer pain or end-of-life care, prolonged use of non-steroidal anti-inflammatory drugs or opioids to manage chronic pain is rarely sustainable.
As the endocannabinoid system is known to control pain at peripheral, spinal, and supraspinal levels, interest in medical use of cannabis is growing.
A proprietary blend of cannabis plant extracts containing delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) as the principal cannabinoids is formulated as an oromucosal spray (USAN name: nabiximols) and standardized to ensure quality, consistency and stability. This review examines evidence for THC:CBD oromucosal spray (nabiximols) in the management of chronic pain conditions.
Cumulative evidence from clinical trials and an exploratory analysis of the German Pain e-Registry suggests that add-on THC:CBD oromucosal spray (nabiximols) may have a role in managing chronic neuropathic pain, although further precise clinical trials are required to draw definitive conclusions.”
https://www.ncbi.nlm.nih.gov/pubmed/32104061
“Smoked Cannabis Proven Effective In Treating Neuropathic Pain.” https://www.sciencedaily.com/releases/2007/10/071024141745.htm
“Marijuana Relieves Chronic Pain, Research Shows” https://www.webmd.com/pain-management/news/20100830/marijuana-relieves-chronic-pain-research-show#1
Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects.
 “Cannabidiol (CBD) is one of the prominent phytocannabinoids found in Cannabis sativa, differentiating from Δ9-tetrahydrocannabinol (THC) for its non-intoxicating profile and its antianxiety/antipsychotic effects. CBD is a multi-target drug whose anti-convulsant properties are supposed to be independent of endocannabinoid receptor CB1 and might be related to several underlying mechanisms, such as antagonism on the orphan GPR55 receptor, regulation of adenosine tone, activation of 5HT1A receptors and modulation of calcium intracellular levels. CBD is a lipophilic compound with low oral bioavailability (6%) due to poor intestinal absorption and high first-pass metabolism. Its exposure parameters are greatly influenced by feeding status (ie, high fat-containing meals). It is mainly metabolized by cytochrome P 450 (CYP) 3A4 and 2C19, which it strongly inhibits.
“Cannabidiol (CBD) is one of the prominent phytocannabinoids found in Cannabis sativa, differentiating from Δ9-tetrahydrocannabinol (THC) for its non-intoxicating profile and its antianxiety/antipsychotic effects. CBD is a multi-target drug whose anti-convulsant properties are supposed to be independent of endocannabinoid receptor CB1 and might be related to several underlying mechanisms, such as antagonism on the orphan GPR55 receptor, regulation of adenosine tone, activation of 5HT1A receptors and modulation of calcium intracellular levels. CBD is a lipophilic compound with low oral bioavailability (6%) due to poor intestinal absorption and high first-pass metabolism. Its exposure parameters are greatly influenced by feeding status (ie, high fat-containing meals). It is mainly metabolized by cytochrome P 450 (CYP) 3A4 and 2C19, which it strongly inhibits.
A proprietary formulation of highly purified, plant-derived CBD has been recently licensed as an adjunctive treatment for Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS), while it is being currently investigated in tuberous sclerosis complex. The regulatory agencies’ approval was granted based on four pivotal double-blind, placebo-controlled, randomized clinical trials (RCTs) on overall 154 DS patients and 396 LGS ones, receiving CBD 10 or 20 mg/kg/day BID as active treatment. The primary endpoint (reduction in monthly seizure frequency) was met by both CBD doses.
Most patients reported adverse events (AEs), generally from mild to moderate and transient, which mainly consisted of somnolence, sedation, decreased appetite, diarrhea and elevation in aminotransferase levels, the last being documented only in subjects on concomitant valproate therapy. The interaction between CBD and clobazam, likely due to CYP2C19 inhibition, might contribute to some AEs, especially somnolence, but also to CBD clinical effectiveness. Cannabidivarin (CBDV), the propyl analogue of CBD, showed anti-convulsant properties in pre-clinical studies, but a plant-derived, purified proprietary formulation of CBDV recently failed the Phase II RCT in patients with uncontrolled focal seizures.”
Localization of cannabinoid and cannabinoid related receptors in the cat gastrointestinal tract.
 “A growing body of literature indicates that activation of cannabinoid receptors may exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.
“A growing body of literature indicates that activation of cannabinoid receptors may exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.
The present study aimed to immunohistochemically investigate the distribution of the canonical cannabinoid receptors CB1 (CB1R) and CB2 (CB2R) and the putative cannabinoid receptors G protein-coupled receptor 55 (GPR55), nuclear peroxisome proliferator-activated receptor alpha (PPARα), transient receptor potential ankyrin 1 (TRPA1), and serotonin receptor 5-HT1a 5-HT1aR) in tissue samples of the gastrointestinal tract of the cat.
CB1R-immunoreactivity (CB1R-IR) was observed in gastric epithelial cells, intestinal enteroendocrine cells (EECs) and goblet cells, lamina propria mast cells (MCs), and enteric neurons. CB2R-IR was expressed by EECs, enterocytes, and macrophages. GPR55-IR was expressed by EECs, macrophages, immunocytes, and MP neurons. PPARα-IR was expressed by immunocytes, smooth muscle cells, and enteroglial cells. TRPA1-IR was expressed by enteric neurons and intestinal goblet cells. 5-HT1a receptor-IR was expressed by gastrointestinal epithelial cells and gastric smooth muscle cells.
Cannabinoid receptors showed a wide distribution in the feline gastrointestinal tract layers. Although not yet confirmed/supported by functional evidences, the present research might represent an anatomical substrate potentially useful to support, in feline species, the therapeutic use of cannabinoids during gastrointestinal inflammatory diseases.”
Targeting Peripherally Restricted Cannabinoid Receptor 1, Cannabinoid Receptor 2, and Endocannabinoid-Degrading Enzymes for the Treatment of Neuropathic Pain Including Neuropathic Orofacial Pain.
 “Neuropathic pain conditions including neuropathic orofacial pain (NOP) are difficult to treat. Contemporary therapeutic agents for neuropathic pain are often ineffective in relieving pain and are associated with various adverse effects. Finding new options for treating neuropathic pain is a major priority in pain-related research.
“Neuropathic pain conditions including neuropathic orofacial pain (NOP) are difficult to treat. Contemporary therapeutic agents for neuropathic pain are often ineffective in relieving pain and are associated with various adverse effects. Finding new options for treating neuropathic pain is a major priority in pain-related research.
Cannabinoid-based therapeutic strategies have emerged as promising new options.
Cannabinoids mainly act on cannabinoid 1 (CB1) and 2 (CB2) receptors, and the former is widely distributed in the brain. The therapeutic significance of cannabinoids is masked by their adverse effects including sedation, motor impairment, addiction and cognitive impairment, which are thought to be mediated by CB1 receptors in the brain. Alternative approaches have been developed to overcome this problem by selectively targeting CB2 receptors, peripherally restricted CB1 receptors and endocannabinoids that may be locally synthesized on demand at sites where their actions are pertinent.
Many preclinical studies have reported that these strategies are effective for treating neuropathic pain and produce no or minimal side effects.
Recently, we observed that inhibition of degradation of a major endocannabinoid, 2-arachydonoylglycerol, can attenuate NOP following trigeminal nerve injury in mice. This review will discuss the above-mentioned alternative approaches that show potential for treating neuropathic pain including NOP.”
Cannabinoid agonists possibly mediate interaction between cholinergic and cannabinoid systems in regulating intestinal inflammation.
 “Inflammatory Bowel Disease (IBD) is idiopathic, chronic and affects the gastrointestinal tract. It results from the association of genetic, environmental and immune deregulation, which culminates in the development and progression of the inflammatory process. In an attempt to reverse colonic inflammation, endogenous systems involved in intestinal physiology are studied and the cholinergic system is fundamental for this process. In addition, this system has anti-inflammatory action in experimental models of IBD. Another important endogenous system in regulating the exacerbated inflammatory response in the gut is mediated by endocannabinoids, which play an important role in restoring bowel functionality after the onset of the inflammatory process. There are several reports in the literature showing the interconnection between the cannabinoid and cholinergic systems in different tissues. Considering that the activation of the cholinergic system stimulates the production of cannabinoid agonists in the intestine, our hypothesis is that the interaction between the muscarinic system and the cannabinoid in the control of intestinal inflammation is mediated by endogenous cannabinoids, since they are stimulated by the activation of muscarinic receptors.”
“Inflammatory Bowel Disease (IBD) is idiopathic, chronic and affects the gastrointestinal tract. It results from the association of genetic, environmental and immune deregulation, which culminates in the development and progression of the inflammatory process. In an attempt to reverse colonic inflammation, endogenous systems involved in intestinal physiology are studied and the cholinergic system is fundamental for this process. In addition, this system has anti-inflammatory action in experimental models of IBD. Another important endogenous system in regulating the exacerbated inflammatory response in the gut is mediated by endocannabinoids, which play an important role in restoring bowel functionality after the onset of the inflammatory process. There are several reports in the literature showing the interconnection between the cannabinoid and cholinergic systems in different tissues. Considering that the activation of the cholinergic system stimulates the production of cannabinoid agonists in the intestine, our hypothesis is that the interaction between the muscarinic system and the cannabinoid in the control of intestinal inflammation is mediated by endogenous cannabinoids, since they are stimulated by the activation of muscarinic receptors.”
https://www.ncbi.nlm.nih.gov/pubmed/32085982
https://www.sciencedirect.com/science/article/abs/pii/S030698771931429X?via%3Dihub
