 “Plant-based therapies date back centuries. Cannabis sativa is one such plant that was used medicinally up until the early part of the 20th century.
“Plant-based therapies date back centuries. Cannabis sativa is one such plant that was used medicinally up until the early part of the 20th century.
Although rich in diverse and interesting phytochemicals, cannabis was largely ignored by the modern scientific community due to its designation as a schedule 1 narcotic and restrictions on access for research purposes. There was renewed interest in the early 1990s when the endocannabinoid system (ECS) was discovered, a complex network of signaling pathways responsible for physiological homeostasis. Two key components of the ECS, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), were identified as the molecular targets of the phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC).
Restrictions on access to cannabis have eased worldwide, leading to a resurgence in interest in the therapeutic potential of cannabis. Much of the focus has been on the two major constituents, Δ9-THC and cannabidiol (CBD). Cannabis contains over 140 phytocannabinoids, although only a handful have been tested for pharmacological activity. Many of these minor cannabinoids potently modulate receptors, ionotropic channels, and enzymes associated with the ECS and show therapeutic potential individually or synergistically with other phytocannabinoids.
The following review will focus on the pharmacological developments of the next generation of phytocannabinoid therapeutics.”
https://pubmed.ncbi.nlm.nih.gov/33356248/
https://pubs.acs.org/doi/10.1021/acs.jnatprod.0c00965


 “Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder with a multifactorial etiology. Latest researches are raising the hypothesis of a link between the onset of the main behavioral symptoms of ASD and the chronic neuroinflammatory condition of the autistic brain; increasing evidence of this connection is shedding light on new possible players in the pathogenesis of ASD.
“Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder with a multifactorial etiology. Latest researches are raising the hypothesis of a link between the onset of the main behavioral symptoms of ASD and the chronic neuroinflammatory condition of the autistic brain; increasing evidence of this connection is shedding light on new possible players in the pathogenesis of ASD. “A significant number of cannabinoids are known to have analgesic and anti-inflammatory properties in various diseases. Due to their presynaptic/terminal location, cannabinoid receptors can inhibit synaptic transmission and have the potential to regulate neurogenic inflammation. Neurogenic inflammation occurs when a noxious signal is detected in the periphery initiating an antidromic axon reflex in the same sensory neurone leading to depolarization of the afferent terminal. Neuropeptides are subsequently released and contribute to vasodilation, plasma extravasation and modulation of immune cells. Endocannabinoids, synthetic cannabinoids and phytocannabinoids can reduce neuroinflammation by inhibiting afferent firing and inflammatory neuropeptide release. Thus, in addition to a direct effect on vascular smooth muscle and inflammatory cells, cannabinoids can reduce inflammation by silencing small diameter neurones. This review examines the neuropharmacological processes involved in regulating antidromic depolarization of afferent nerve terminals by cannabinoids and the control of neurogenic inflammation in different diseases.”
“A significant number of cannabinoids are known to have analgesic and anti-inflammatory properties in various diseases. Due to their presynaptic/terminal location, cannabinoid receptors can inhibit synaptic transmission and have the potential to regulate neurogenic inflammation. Neurogenic inflammation occurs when a noxious signal is detected in the periphery initiating an antidromic axon reflex in the same sensory neurone leading to depolarization of the afferent terminal. Neuropeptides are subsequently released and contribute to vasodilation, plasma extravasation and modulation of immune cells. Endocannabinoids, synthetic cannabinoids and phytocannabinoids can reduce neuroinflammation by inhibiting afferent firing and inflammatory neuropeptide release. Thus, in addition to a direct effect on vascular smooth muscle and inflammatory cells, cannabinoids can reduce inflammation by silencing small diameter neurones. This review examines the neuropharmacological processes involved in regulating antidromic depolarization of afferent nerve terminals by cannabinoids and the control of neurogenic inflammation in different diseases.” “Leukocytes are part of the tumor microenvironment (TME) and are critical determinants of tumor progression. Because of the immunoregulatory properties of cannabinoids, the endocannabinoid system (ECS) may have an important role in shaping the TME.
“Leukocytes are part of the tumor microenvironment (TME) and are critical determinants of tumor progression. Because of the immunoregulatory properties of cannabinoids, the endocannabinoid system (ECS) may have an important role in shaping the TME.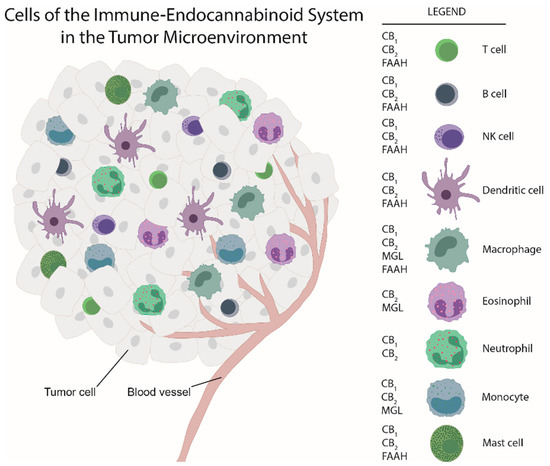
 “Alzheimer’s disease (AD) is characterized by structural damage, death, and functional disruption of cholinergic neurons (ChNs) as a result of intracellular amyloid-β (Aβ) aggregation, extracellular neuritic plaques, and hyperphosphorylation of protein tau (p-Tau) overtime.
“Alzheimer’s disease (AD) is characterized by structural damage, death, and functional disruption of cholinergic neurons (ChNs) as a result of intracellular amyloid-β (Aβ) aggregation, extracellular neuritic plaques, and hyperphosphorylation of protein tau (p-Tau) overtime. “Chronic pelvic pain (CPP) affects up to 15% of women in the United States. The endocannabinoid system is a potential pharmacological target for pelvic pain as cannabinoid receptors are highly expressed in the uterus and other nonreproductive tissues.
“Chronic pelvic pain (CPP) affects up to 15% of women in the United States. The endocannabinoid system is a potential pharmacological target for pelvic pain as cannabinoid receptors are highly expressed in the uterus and other nonreproductive tissues. “Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
“Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant. “Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS.
“Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS.