“Cannabinoid-based medications possess unique multimodal analgesic mechanisms of action, modulating diverse pain targets.
Cannabinoids are classified based on their origin into three categories: endocannabinoids (present endogenously in human tissues), phytocannabinoids (plant derived) and synthetic cannabinoids (pharmaceutical). Cannabinoids exert an analgesic effect, peculiarly in hyperalgesia, neuropathic pain and inflammatory states.
Endocannabinoids are released on demand from postsynaptic terminals and travels retrograde to stimulate cannabinoids receptors on presynaptic terminals, inhibiting the release of excitatory neurotransmitters. Cannabinoids (endogenous and phytocannabinoids) produce analgesia by interacting with cannabinoids receptors type 1 and 2 (CB1 and CB2), as well as putative non-CB1/CB2 receptors; G protein-coupled receptor 55, and transient receptor potential vanilloid type-1. Moreover, they modulate multiple peripheral, spinal and supraspinal nociception pathways.
Cannabinoids-opioids cross-modulation and synergy contribute significantly to tolerance and antinociceptive effects of cannabinoids. This narrative review evaluates cannabinoids’ diverse mechanisms of action as it pertains to nociception modulation relevant to the practice of anesthesiologists and pain medicine physicians.”
https://pubmed.ncbi.nlm.nih.gov/33239391/
https://rapm.bmj.com/content/early/2020/11/24/rapm-2020-102114

 “Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies.
“Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies. “The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.
“The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.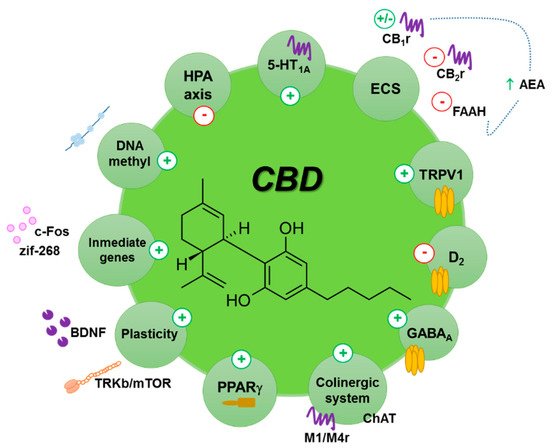
 “The COVID-19 pandemic caused by SARS-CoV-2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune-inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably.
“The COVID-19 pandemic caused by SARS-CoV-2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune-inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably.
 “Cannabis has been used as a medicine for millennia. Prohibition in the mid-20th century precluded early scientific investigation.
“Cannabis has been used as a medicine for millennia. Prohibition in the mid-20th century precluded early scientific investigation. “Medical cannabis and individual cannabinoids, such as tetrahydrocannabinol (THC) and cannabidiol (CBD), are receiving growing attention in both the media and the scientific literature. The Cannabis plant, however, produces over 100 different cannabinoids, and cannabigerol (CBG) serves as the precursor molecule for the most abundant phytocannabinoids.
“Medical cannabis and individual cannabinoids, such as tetrahydrocannabinol (THC) and cannabidiol (CBD), are receiving growing attention in both the media and the scientific literature. The Cannabis plant, however, produces over 100 different cannabinoids, and cannabigerol (CBG) serves as the precursor molecule for the most abundant phytocannabinoids. “The endocannabinoid (eCB) system encompasses the eCBs anandamide and 2-arachidonoylglycerol, their anabolic/catabolic enzymes, and the cannabinoid CB1 and CB2 receptors. Its expansion to include several eCB-like lipid mediators, their metabolic enzymes, and their molecular targets, forms the endocannabinoidome (eCBome).
“The endocannabinoid (eCB) system encompasses the eCBs anandamide and 2-arachidonoylglycerol, their anabolic/catabolic enzymes, and the cannabinoid CB1 and CB2 receptors. Its expansion to include several eCB-like lipid mediators, their metabolic enzymes, and their molecular targets, forms the endocannabinoidome (eCBome). “Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown.
“Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown. “(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants.
“(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants.