“Alzheimer’s disease (AD) is characterized by structural damage, death, and functional disruption of cholinergic neurons (ChNs) as a result of intracellular amyloid-β (Aβ) aggregation, extracellular neuritic plaques, and hyperphosphorylation of protein tau (p-Tau) overtime.
“Alzheimer’s disease (AD) is characterized by structural damage, death, and functional disruption of cholinergic neurons (ChNs) as a result of intracellular amyloid-β (Aβ) aggregation, extracellular neuritic plaques, and hyperphosphorylation of protein tau (p-Tau) overtime.
Objective: To evaluate the effect of the synthetic cannabinoid CP55940 (CP) on PSEN1 E280A cholinergic-like nerve cells (PSEN1 ChLNs)-a natural model of familial AD.
Results: CP in the presence of both inverse agonists (hereafter SR) almost completely inhibits the aggregation of intracellular sAβPPβf and p-Tau, increases ΔΨm, decreases oxidation of DJ-1Cys106-SH residue, and blocks the activation of c-Jun, p53, PUMA, and caspase-3 independently of CB1Rs signaling in mutant ChLNs. CP also inhibits the generation of reactive oxygen species partially dependent on CB1Rs. Although CP reduced extracellular Aβ 42, it was unable to reverse the Ca2 + influx dysregulation as a response to acetylcholine stimuli in mutant ChLNs. Exposure to anti-Aβ antibody 6E10 (1:300) in the absence or presence of SR plus CP completely recovered transient [Ca2 +]i signal as a response to acetylcholine in mutant ChLNs.
Conclusion: Taken together our findings suggest that the combination of cannabinoids, CB1Rs inverse agonists, and anti-Aβ antibodies might be a promising therapeutic approach for the treatment of familial AD.”
https://pubmed.ncbi.nlm.nih.gov/33252082/
“It is therefore proposed that combinations of cannabinoids, anti-Aβ 42 antibodies (e.g., crenezumab), and CB1 inverse agonists might be a promising multi-target drugs for therapy in the early treatment of FAD PSEN 1 E280A ChLNs neurodegeneration.”
https://content.iospress.com/articles/journal-of-alzheimers-disease/jad201045

 “Chronic pelvic pain (CPP) affects up to 15% of women in the United States. The endocannabinoid system is a potential pharmacological target for pelvic pain as cannabinoid receptors are highly expressed in the uterus and other nonreproductive tissues.
“Chronic pelvic pain (CPP) affects up to 15% of women in the United States. The endocannabinoid system is a potential pharmacological target for pelvic pain as cannabinoid receptors are highly expressed in the uterus and other nonreproductive tissues. “Opioid misuse and overuse has contributed to a widespread overdose crisis and many patients and physicians are considering medical cannabis to support opioid tapering and chronic pain control. Using a five-step modified Delphi process, we aimed to develop consensus-based recommendations on: 1) when and how to safely initiate and titrate cannabinoids in the presence of opioids, 2) when and how to safely taper opioids in the presence of cannabinoids, and 3) how to monitor patients and evaluate outcomes when treating with opioids and cannabinoids.
“Opioid misuse and overuse has contributed to a widespread overdose crisis and many patients and physicians are considering medical cannabis to support opioid tapering and chronic pain control. Using a five-step modified Delphi process, we aimed to develop consensus-based recommendations on: 1) when and how to safely initiate and titrate cannabinoids in the presence of opioids, 2) when and how to safely taper opioids in the presence of cannabinoids, and 3) how to monitor patients and evaluate outcomes when treating with opioids and cannabinoids. “Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy.
“Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy. “Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
“Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant. “Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS.
“Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS. “Cannabidiol (CBD) has anti-tumorigenic activity. However, the anti-cancer effect of CBD on head and neck squamous cell carcinoma (HNSCC) remains unclear. The cytotoxicity of CBD on HNSCC was analyzed using cell survival and colony-forming assays in vitro.
“Cannabidiol (CBD) has anti-tumorigenic activity. However, the anti-cancer effect of CBD on head and neck squamous cell carcinoma (HNSCC) remains unclear. The cytotoxicity of CBD on HNSCC was analyzed using cell survival and colony-forming assays in vitro. “Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies.
“Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies. “The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.
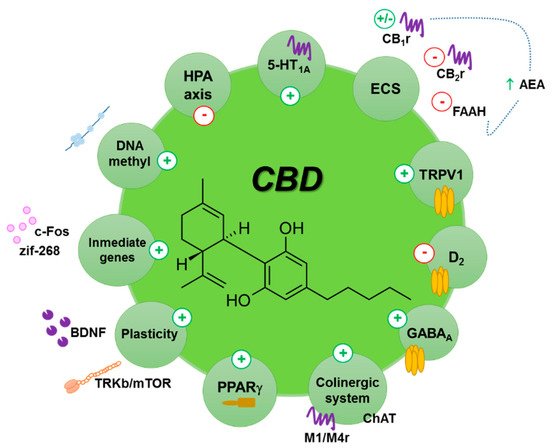
“The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.