“The cannabis plant has been widely researched for many therapeutic indications and found to be effective in many chronic conditions such as epilepsy, neuropathic or chronic pain and more. However, biased opinion against compounds of the plant, regulatory as well as compounding challenges have led to very few approved medicinal products. Those formulations which are approved are dosed several times a day, creating an unmet need for controlled release (CR) formulations of cannabinoids. Conventional CR formulations rely on prolonged absorption including the colon. The purpose of this work is to investigate regional absorption of major cannabinoids THC and CBD from the colon and develop a suitable CR formulation. As hypothesized by researchers, THC and CBD have poor absorption from the colon compared to small intestine, suggesting that these compounds have a narrow absorption window. The suggested formulation examined in-vitro was a floating gastro retentive tablet based on egg albumin matrix, gas generating agents and surfactants. In-vivo investigation of CBD containing formulation in the freely moving rat model proved a prolonged absorption phase with a substantial increase in bioavailability compared to CBD solution. The findings of this paper answer a crucial question regarding potential application of CR dosage forms for cannabinoids and shed light on the regional intestinal absorption of these compounds. Ultimately, these results cement the way for future development of cannabinoid gastro retentive dosage forms.”
“The cannabis plant has been widely researched for many therapeutic indications and found to be effective in many chronic conditions such as epilepsy, neuropathic or chronic pain and more. However, biased opinion against compounds of the plant, regulatory as well as compounding challenges have led to very few approved medicinal products. Those formulations which are approved are dosed several times a day, creating an unmet need for controlled release (CR) formulations of cannabinoids. Conventional CR formulations rely on prolonged absorption including the colon. The purpose of this work is to investigate regional absorption of major cannabinoids THC and CBD from the colon and develop a suitable CR formulation. As hypothesized by researchers, THC and CBD have poor absorption from the colon compared to small intestine, suggesting that these compounds have a narrow absorption window. The suggested formulation examined in-vitro was a floating gastro retentive tablet based on egg albumin matrix, gas generating agents and surfactants. In-vivo investigation of CBD containing formulation in the freely moving rat model proved a prolonged absorption phase with a substantial increase in bioavailability compared to CBD solution. The findings of this paper answer a crucial question regarding potential application of CR dosage forms for cannabinoids and shed light on the regional intestinal absorption of these compounds. Ultimately, these results cement the way for future development of cannabinoid gastro retentive dosage forms.”
https://www.ncbi.nlm.nih.gov/pubmed/32422168
https://www.sciencedirect.com/science/article/abs/pii/S0939641120301375?via%3Dihub

 “Two patient case reports are presented describing the use of cannabidiol (CBD) for the symptomatic relief of a lumbar compression fracture and in the mitigation of thoracic discomfort and dysesthesia secondary to a surgically resected meningioma.
“Two patient case reports are presented describing the use of cannabidiol (CBD) for the symptomatic relief of a lumbar compression fracture and in the mitigation of thoracic discomfort and dysesthesia secondary to a surgically resected meningioma. “The study documented here was aimed to find the molecular interactions of some of the cannabinoid constituents of
“The study documented here was aimed to find the molecular interactions of some of the cannabinoid constituents of 
 “The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival.
“The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival. “There is increasing interest in the use of purified
“There is increasing interest in the use of purified 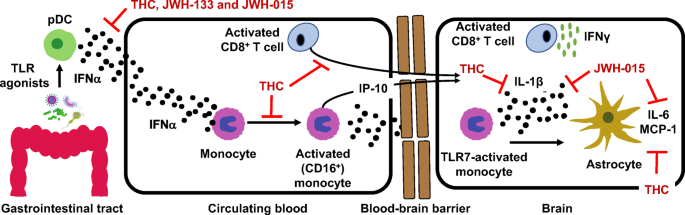

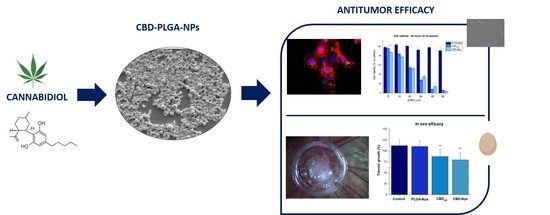
 “The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
“The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.