 “For patients with chronic, non-cancer pain, traditional pain-relieving medications include opioids, which have shown benefits but are associated with increased risks of addiction and adverse effects.
“For patients with chronic, non-cancer pain, traditional pain-relieving medications include opioids, which have shown benefits but are associated with increased risks of addiction and adverse effects.
Medical cannabis has emerged as a treatment alternative for managing these patients and there has been a rise in the number of randomized clinical trials in recent years; therefore, a systematic review of the evidence was warranted.
RESULTS:
Thirty-six trials (4006 participants) were included, examining smoked cannabis (4 trials), oromucosal cannabis sprays (14 trials), and oral cannabinoids (18 trials). Compared with placebo, cannabinoids showed a significant reduction in pain which was greatest with treatment duration of 2 to 8 weeks (weighted mean difference on a 0-10 pain visual analogue scale -0.68, 95% confidence interval [CI], -0.96 to -0.40, I 2 = 8%, P < .00001; n = 16 trials). When stratified by route of administration, pain condition, and type of cannabinoids, oral cannabinoids had a larger reduction in pain compared with placebo relative to oromucosal and smoked formulations but the difference was not significant (P[interaction] > .05 in all the 3 durations of treatment); cannabinoids had a smaller reduction in pain due to multiple sclerosis compared with placebo relative to other neuropathic pain (P[interaction] = .05) within 2 weeks and the difference was not significant relative to pain due to rheumatic arthritis; nabilone had a greater reduction in pain compared with placebo relative to other types of cannabinoids longer than 2 weeks of treatment but the difference was not significant (P[interaction] > .05). Serious AEs were rare, and similar across the cannabinoid (74 out of 2176, 3.4%) and placebo groups (53 out of 1640, 3.2%). There was an increased risk of non-serious AEs with cannabinoids compared with placebo.
CONCLUSIONS:
There was moderate evidence to support cannabinoids in treating chronic, non-cancer pain at 2 weeks. Similar results were observed at later time points, but the confidence in effect is low. There is little evidence that cannabinoids increase the risk of experiencing serious AEs, although non-serious AEs may be common in the short-term period following use.”
https://www.ncbi.nlm.nih.gov/pubmed/32127750
https://journals.sagepub.com/doi/10.1177/1179544120906461
 “Cannabis has been considered as a therapeutic strategy to control intractable epilepsy.
“Cannabis has been considered as a therapeutic strategy to control intractable epilepsy.
 “The
“The  “Phytocannabinoids (pCBs) are a large family of meroterpenoids isolated from the plant Cannabis sativa. Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best investigated phytocannabinoids due to their relative abundance and interesting bioactivity profiles. In addition to various targets, THC and CBD are also well-known agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor involved in energy homeostasis and lipid metabolism. In the search of new pCBs potentially acting as PPARγ agonists, we identified cannabimovone (CBM), a structurally unique abeo-menthane pCB, as a novel PPARγ modulator via a combined computational and experimental approach. The ability of CBM to act as dual PPARγ/α agonist was also evaluated. Computational studies suggested a different binding mode toward the two isoforms, with the compound able to recapitulate the pattern of H-bonds of a canonical agonist only in the case of PPARγ. Luciferase assays confirmed the computational results, showing a selective activation of PPARγ by CBM in the low micromolar range. CBM promoted the expression of PPARγ target genes regulating the adipocyte differentiation and prevented palmitate-induced insulin signaling impairment. Altogether, these results candidate CBM as a novel bioactive compound potentially useful for the treatment of insulin resistance-related disorders.”
“Phytocannabinoids (pCBs) are a large family of meroterpenoids isolated from the plant Cannabis sativa. Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best investigated phytocannabinoids due to their relative abundance and interesting bioactivity profiles. In addition to various targets, THC and CBD are also well-known agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor involved in energy homeostasis and lipid metabolism. In the search of new pCBs potentially acting as PPARγ agonists, we identified cannabimovone (CBM), a structurally unique abeo-menthane pCB, as a novel PPARγ modulator via a combined computational and experimental approach. The ability of CBM to act as dual PPARγ/α agonist was also evaluated. Computational studies suggested a different binding mode toward the two isoforms, with the compound able to recapitulate the pattern of H-bonds of a canonical agonist only in the case of PPARγ. Luciferase assays confirmed the computational results, showing a selective activation of PPARγ by CBM in the low micromolar range. CBM promoted the expression of PPARγ target genes regulating the adipocyte differentiation and prevented palmitate-induced insulin signaling impairment. Altogether, these results candidate CBM as a novel bioactive compound potentially useful for the treatment of insulin resistance-related disorders.”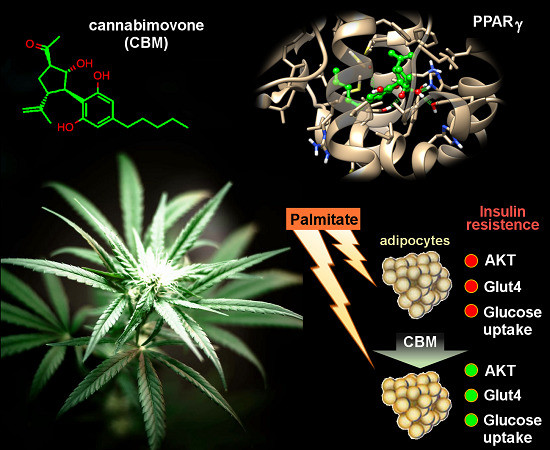
 “Cannabinoids are extracted from Cannabis sativa L. and are used for a variety of medicinal purposes.
“Cannabinoids are extracted from Cannabis sativa L. and are used for a variety of medicinal purposes. “Psoriasis is a chronic inflammatory skin disease characterized by dysregulated keratinocyte differentiation, but oxidative stress also plays an important role in the pathogenesis of this disease.
“Psoriasis is a chronic inflammatory skin disease characterized by dysregulated keratinocyte differentiation, but oxidative stress also plays an important role in the pathogenesis of this disease. “Multiple sclerosis (MS) is a highly symptomatic disease, with a wide range of disabilities affecting many bodily functions, even in younger persons with a short disease history.
“Multiple sclerosis (MS) is a highly symptomatic disease, with a wide range of disabilities affecting many bodily functions, even in younger persons with a short disease history. “Over the past decade, patients, families, and medical cannabis advocates have campaigned in many countries to allow patients to use cannabis preparations to treat the symptoms of serious illnesses that have not responded to conventional treatment.
“Over the past decade, patients, families, and medical cannabis advocates have campaigned in many countries to allow patients to use cannabis preparations to treat the symptoms of serious illnesses that have not responded to conventional treatment. “For patients with chronic, non-cancer pain, traditional pain-relieving medications include opioids, which have shown benefits but are associated with increased risks of addiction and adverse effects.
“For patients with chronic, non-cancer pain, traditional pain-relieving medications include opioids, which have shown benefits but are associated with increased risks of addiction and adverse effects. “While medical and recreational cannabis use is becoming more frequent among older adults, the neurocognitive consequences of cannabis use in this age group are unclear. The aim of this literature review was to synthesize and evaluate the current knowledge on the association of cannabis use during older-adulthood with cognitive function and brain aging.
“While medical and recreational cannabis use is becoming more frequent among older adults, the neurocognitive consequences of cannabis use in this age group are unclear. The aim of this literature review was to synthesize and evaluate the current knowledge on the association of cannabis use during older-adulthood with cognitive function and brain aging.