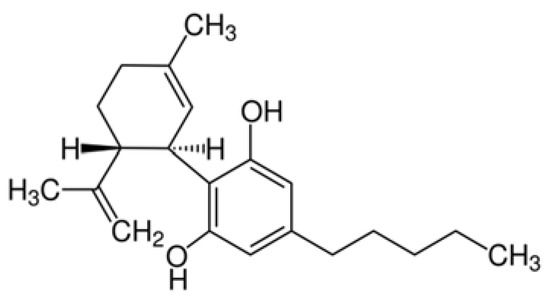
“Preclinical studies have shown cannabidiol is protective in models of ischemic stroke. Based on results from our recent systematic review, we investigated the effects of two promising neuroprotective phytocannabinoids, cannabigerol (CBG) and cannabidivarin (CBDV), on cells of the blood-brain barrier (BBB), namely human brain microvascular endothelial cells (HBMECs), pericytes, and astrocytes.
“Preclinical studies have shown cannabidiol is protective in models of ischemic stroke. Based on results from our recent systematic review, we investigated the effects of two promising neuroprotective phytocannabinoids, cannabigerol (CBG) and cannabidivarin (CBDV), on cells of the blood-brain barrier (BBB), namely human brain microvascular endothelial cells (HBMECs), pericytes, and astrocytes.
Results: In astrocytes CBG and CBDV attenuated levels of interleukin-6 (IL-6) and lactate dehydrogenase (LDH), whereas CBDV (10 nM-10 μM) also decreased vascular endothelial growth factor (VEGF) secretion. CBDV (300 nM-10 μM) attenuated levels of monocyte chemoattractant protein (MCP)-1 in HBMECs. In astrocytes, CBG decreased levels of DNA damage proteins, including p53, whereas CBDV increased levels of DNA damage markers. Antagonists for CB1, CB2, PPAR-γ, PPAR-α, 5-HT1A, and TRPV1 had no effect on CBG (3 μM) or CBDV (1 μM)-mediated decreases in LDH in astrocytes. GPR55 and GPR18 were partially implicated in the effects of CBDV, but no molecular target was identified for CBG.
Conclusions: We show that CBG and CBDV were protective against OG mediated injury in three different cells that constitute the BBB, modulating different hallmarks of ischemic stroke pathophysiology. These data enhance our understanding of the protective effects of CBG and CBDV and warrant further investigation into these compounds in ischemic stroke. Future studies should identify other possible neuroprotective effects of CBG and CBDV and their corresponding mechanisms of action.”
https://pubmed.ncbi.nlm.nih.gov/33998890/
“This study provides novel data on the neuroprotective and anti-inflammatory properties of CBG and CBDV in an in vitro model of IR. These data, together with evidence from other studies, corroborate the protective properties of these compounds and further studies are needed to elucidate the mechanism of action of CBG and CBDV and whether they can modulate BBB permeability in more clinically relevant in vivo models of ischemic stroke. There is lack of effective treatments for ischemic stroke, a condition that will increase in prevalence in coming years, to which cannabinoids may offer a unique therapeutic strategy.”

 “An observational research design was used to evaluate which types of commonly labeled Cannabis flower product characteristics are associated with changes in momentary feelings of distress-related symptoms.
“An observational research design was used to evaluate which types of commonly labeled Cannabis flower product characteristics are associated with changes in momentary feelings of distress-related symptoms. “Cannabinoids are a family of heterogeneous compounds that mostly interact with receptors eliciting several physiological effects both in the central and peripheral nervous systems and in peripheral organs. They exert anticancer action by modulating signaling pathways involved in cancer progression; furthermore, the effects induced by their use depend on both the type of tumor and their action on the components of the endocannabinoid system. This review will explore the mechanism of action of the cannabinoids in signaling pathways involved in cancer proliferation, neovascularisation, migration, invasion, metastasis, and tumor angiogenesis.”
“Cannabinoids are a family of heterogeneous compounds that mostly interact with receptors eliciting several physiological effects both in the central and peripheral nervous systems and in peripheral organs. They exert anticancer action by modulating signaling pathways involved in cancer progression; furthermore, the effects induced by their use depend on both the type of tumor and their action on the components of the endocannabinoid system. This review will explore the mechanism of action of the cannabinoids in signaling pathways involved in cancer proliferation, neovascularisation, migration, invasion, metastasis, and tumor angiogenesis.”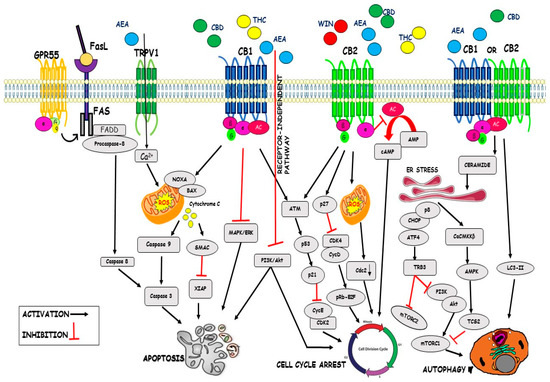
 “Agents targeting the endocannabinoid system (ECS) have gained attention as potential cancer treatments. Given recent evidence that cannabinoid receptor 2 (CB2R) regulates lymphocyte development and inflammation, we performed studies on CB2R in the immune response against melanoma. Analysis of The Cancer Genome Atlas (TCGA) data revealed a strong positive correlation between CB2R expression and survival, as well as B cell infiltration in human melanoma. In a murine melanoma model, CB2R expression reduced the growth of melanoma as well as the B cell frequencies in the tumor microenvironment (TME), compared to CB2R-deficient mice. In depth analysis of tumor-infiltrating B cells using single-cell RNA sequencing suggested a less differentiated phenotype in tumors from
“Agents targeting the endocannabinoid system (ECS) have gained attention as potential cancer treatments. Given recent evidence that cannabinoid receptor 2 (CB2R) regulates lymphocyte development and inflammation, we performed studies on CB2R in the immune response against melanoma. Analysis of The Cancer Genome Atlas (TCGA) data revealed a strong positive correlation between CB2R expression and survival, as well as B cell infiltration in human melanoma. In a murine melanoma model, CB2R expression reduced the growth of melanoma as well as the B cell frequencies in the tumor microenvironment (TME), compared to CB2R-deficient mice. In depth analysis of tumor-infiltrating B cells using single-cell RNA sequencing suggested a less differentiated phenotype in tumors from 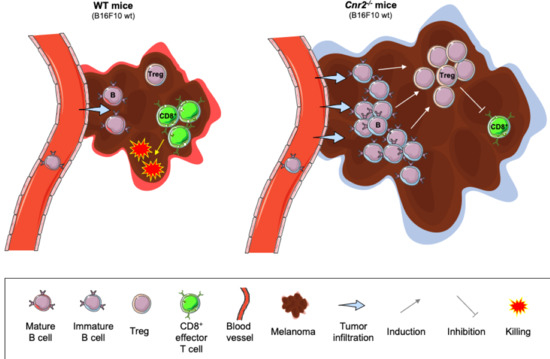
 “Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB
“Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB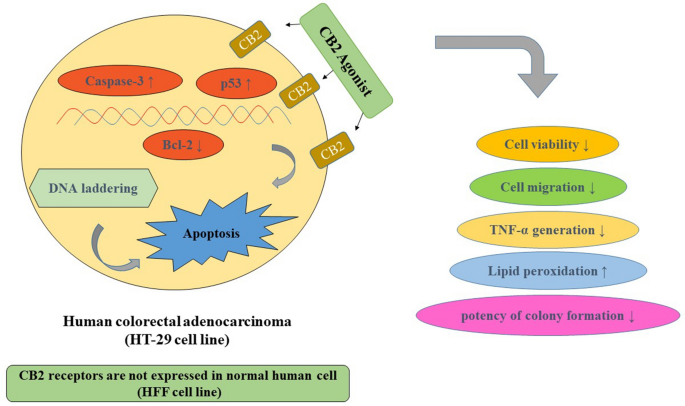
 “In recent years, evidence has accumulated that cannabinoids-especially the non-psychoactive compound, cannabidiol (CBD)-possess promising medical and pharmacological activities that might qualify them as potential anti-tumor drugs. This review is based on multiple studies summarizing different mechanisms for how CBD can target tumor cells including cannabinoid receptors or other constituents of the endocannabinoid system, and their complex activation of biological systems that results in the inhibition of tumor growth. CBD also participates in anti-inflammatory activities which are related to tumor progression, as demonstrated in preclinical models. Although the numbers of clinical trials and tested tumor entities are limited, there is clear evidence that CBD has anti-tumor efficacy and is well tolerated in human cancer patients. In summary, it appears that CBD has potential as a neoadjuvant and/or adjuvant drug in therapy for cancer.”
“In recent years, evidence has accumulated that cannabinoids-especially the non-psychoactive compound, cannabidiol (CBD)-possess promising medical and pharmacological activities that might qualify them as potential anti-tumor drugs. This review is based on multiple studies summarizing different mechanisms for how CBD can target tumor cells including cannabinoid receptors or other constituents of the endocannabinoid system, and their complex activation of biological systems that results in the inhibition of tumor growth. CBD also participates in anti-inflammatory activities which are related to tumor progression, as demonstrated in preclinical models. Although the numbers of clinical trials and tested tumor entities are limited, there is clear evidence that CBD has anti-tumor efficacy and is well tolerated in human cancer patients. In summary, it appears that CBD has potential as a neoadjuvant and/or adjuvant drug in therapy for cancer.”