 “Chronic ethanol abuse can lead to harmful consequences for the heart, resulting in systolic dysfunction, variability in the heart rate, arrhythmia, and cardiac remodelling. However, the precise molecular mechanism responsible for ethanol-induced cardiomyopathy is poorly understood. In this regard, the present study aimed to describe the RIP1/RIP3/MLKL-mediated necroptotic cell death that may be involved in ethanol-induced cardiomyopathy and characterize CBR-mediated effects on the signalling pathway and myocardial injury.
“Chronic ethanol abuse can lead to harmful consequences for the heart, resulting in systolic dysfunction, variability in the heart rate, arrhythmia, and cardiac remodelling. However, the precise molecular mechanism responsible for ethanol-induced cardiomyopathy is poorly understood. In this regard, the present study aimed to describe the RIP1/RIP3/MLKL-mediated necroptotic cell death that may be involved in ethanol-induced cardiomyopathy and characterize CBR-mediated effects on the signalling pathway and myocardial injury.
We performed an ethanol vapour administration experiment to analyse the effects of ethanol on cardiac structure and function in male C57BL/6J mice. Ethanol induced a significant decline in the cardiac structure and function, as evidenced by a decline in ejection fraction and fractional shortening, and an increase in serum Creatine Kinase levels, myocardial collagen content, and inflammatory reaction. Furthermore, ethanol also upregulated the expression levels of necroptosis-related markers such as p-RIP1, p-RIP3, and p-MLKL in the myocardium. Nec-1 treatment exerted significant cardioprotective effects by salvaging the heart tissue, improving the cardiac function, and mitigating inflammation and necroptosis.
In addition, ethanol abuse caused an imbalance in the endocannabinoid system and regulated two cannabinoid receptors (CB1R and CB2R) in the myocardium. Treatment with selective CB2R agonists, JWH-133 or AM1241, markedly improved the cardiac dysfunction and reduced the ethanol-induced necroptosis in the myocardium.
Altogether, our data provide evidence that ethanol abuse-induced cardiotoxicity can possibly be attributed to the RIP1/RIP3/MLKL-mediated necroptosis. Moreover, pharmacological activation of CB2R may represent a new cardioprotective strategy against ethanol-induced cardiotoxicity.”
https://pubmed.ncbi.nlm.nih.gov/32681290/
https://link.springer.com/article/10.1007%2Fs11010-020-03828-1

 “Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
“Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.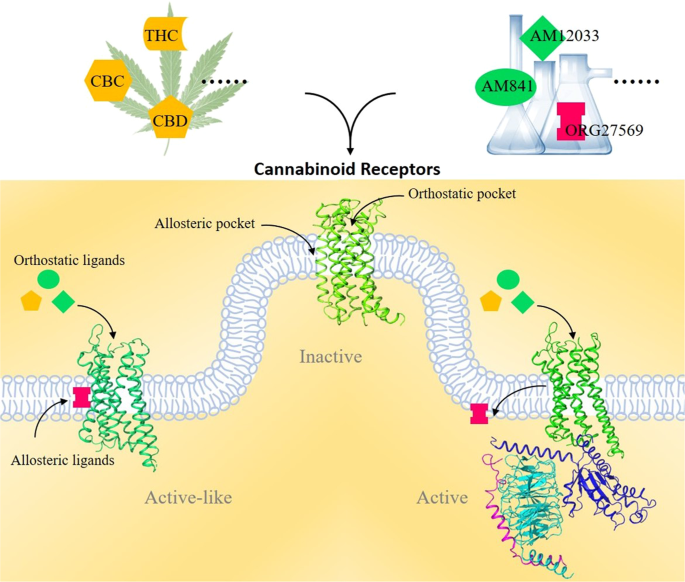
 “HIV is associated with disruptions in cognition and brain function.
“HIV is associated with disruptions in cognition and brain function. “In the last few years research into Cannabis and its constituent phytocannabinoids has burgeoned, particularly in the potential application of novel cannabis phytochemicals for the treatment of diverse illnesses related to neurodegeneration and dementia, including Alzheimer’s (AD), Parkinson’s (PD) and Huntington’s disease (HD). To date, these neurological diseases have mostly relied on symptomatological management. However, with an aging population globally, the search for more efficient and disease-modifying treatments that could delay or mitigate disease progression is imperative. In this context, this review aims to present a state of art in the research with cannabinoids and novel cannabinoid-based drug candidates that have been emerged as novel promising alternatives for drug development and innovation in the therapeutics of a number of diseases, especially those related to CNS-disturbance and impairment.”
“In the last few years research into Cannabis and its constituent phytocannabinoids has burgeoned, particularly in the potential application of novel cannabis phytochemicals for the treatment of diverse illnesses related to neurodegeneration and dementia, including Alzheimer’s (AD), Parkinson’s (PD) and Huntington’s disease (HD). To date, these neurological diseases have mostly relied on symptomatological management. However, with an aging population globally, the search for more efficient and disease-modifying treatments that could delay or mitigate disease progression is imperative. In this context, this review aims to present a state of art in the research with cannabinoids and novel cannabinoid-based drug candidates that have been emerged as novel promising alternatives for drug development and innovation in the therapeutics of a number of diseases, especially those related to CNS-disturbance and impairment.” “In this work, we evaluated, for the first time, the antitumor effect of cannabidiol (CBD) as monotherapy and in combination with conventional chemotherapeutics in ovarian cancer and developed PLGA-microparticles as CBD carriers to optimize its anticancer activity.
“In this work, we evaluated, for the first time, the antitumor effect of cannabidiol (CBD) as monotherapy and in combination with conventional chemotherapeutics in ovarian cancer and developed PLGA-microparticles as CBD carriers to optimize its anticancer activity.
 “In recent years, the role of the endocannabinoid system (ECS) in various cardiovascular conditions has been a subject of great interest. The ECS is composed of cannabinoid receptors, their endogenous ligands, also known as endocannabinoids, and enzymes responsible for the synthesis and degradation of endocannabinoids.
“In recent years, the role of the endocannabinoid system (ECS) in various cardiovascular conditions has been a subject of great interest. The ECS is composed of cannabinoid receptors, their endogenous ligands, also known as endocannabinoids, and enzymes responsible for the synthesis and degradation of endocannabinoids. “So far, no vaccine has been successfully developed and there is no effective treatment of COVID-19.
“So far, no vaccine has been successfully developed and there is no effective treatment of COVID-19. “New neuroprotective treatments of natural origin are being investigated. Both, plant extracts and isolated compounds have shown bioactive effects.
“New neuroprotective treatments of natural origin are being investigated. Both, plant extracts and isolated compounds have shown bioactive effects.
 “Thirty years ago, the discovery of a cannabinoid (CB) receptor that interacts with the psychoactive compound in Cannabis led to the identification of anandamide, an endogenous receptor ligand or endocannabinoid. Research on endocannabinoids has since exploded, and additional receptors along with their lipid mediators and signaling pathways continue to be revealed. Specifically, in humans, the release of endocannabinoids from membrane lipids occurs on demand and the signaling process is rapidly attenuated by the breakdown of the ligand suggesting a tight regulation of the endocannabinoid system (ECS). Additionally, the varying distribution of CB receptors between the central nervous system and other tissues allows for the ECS to participate in a wide range of cognitive and physiological processes. Select plant-derived ‘phyto’cannabinoids such as Δ-9-tetrahydrocannabinol (Δ9-THC) bind to the CB receptors and trigger the ECS, and in the case of Δ9-THC, while it has therapeutic value, can also produce detrimental effects. Current research is aimed at the identification of additional phytocannabinoids with minimal psychotropic effects with potential for therapeutic development. Although decades of research on the ECS and its components have expanded our understanding of the mechanisms and implications of endocannabinoid signaling in mammals, it continues to evolve. Here, we provide a brief overview of the ECS and its overlap with other related lipid-mediated signaling pathways.”
“Thirty years ago, the discovery of a cannabinoid (CB) receptor that interacts with the psychoactive compound in Cannabis led to the identification of anandamide, an endogenous receptor ligand or endocannabinoid. Research on endocannabinoids has since exploded, and additional receptors along with their lipid mediators and signaling pathways continue to be revealed. Specifically, in humans, the release of endocannabinoids from membrane lipids occurs on demand and the signaling process is rapidly attenuated by the breakdown of the ligand suggesting a tight regulation of the endocannabinoid system (ECS). Additionally, the varying distribution of CB receptors between the central nervous system and other tissues allows for the ECS to participate in a wide range of cognitive and physiological processes. Select plant-derived ‘phyto’cannabinoids such as Δ-9-tetrahydrocannabinol (Δ9-THC) bind to the CB receptors and trigger the ECS, and in the case of Δ9-THC, while it has therapeutic value, can also produce detrimental effects. Current research is aimed at the identification of additional phytocannabinoids with minimal psychotropic effects with potential for therapeutic development. Although decades of research on the ECS and its components have expanded our understanding of the mechanisms and implications of endocannabinoid signaling in mammals, it continues to evolve. Here, we provide a brief overview of the ECS and its overlap with other related lipid-mediated signaling pathways.”
 “Highly purified cannabidiol (CBD) (approved as Epidiolex® in the United States) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut or Dravet syndrome in four randomized controlled trials. CBD possesses affinity for many target classes with functional effects relevant to the pathophysiology of many disease types, including epilepsy.
“Highly purified cannabidiol (CBD) (approved as Epidiolex® in the United States) has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut or Dravet syndrome in four randomized controlled trials. CBD possesses affinity for many target classes with functional effects relevant to the pathophysiology of many disease types, including epilepsy.